Match the solutions to the descriptions of the freezing points. Clear All One mole of the nonelectrolyte C, H O dissolved in 1000 g H,O highest freezing point One mole of the ionic intermediate freezing point compound Nag PO4 dissolved in 1000 g H2O One mole of the ionic compound CUSO, dissolved in 1000 g H2 O lowest freezing point
Match the solutions to the descriptions of the freezing points. Clear All One mole of the nonelectrolyte C, H O dissolved in 1000 g H,O highest freezing point One mole of the ionic intermediate freezing point compound Nag PO4 dissolved in 1000 g H2O One mole of the ionic compound CUSO, dissolved in 1000 g H2 O lowest freezing point
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter7: Sollutions And Colloids
Section: Chapter Questions
Problem 7.67E
Related questions
Question
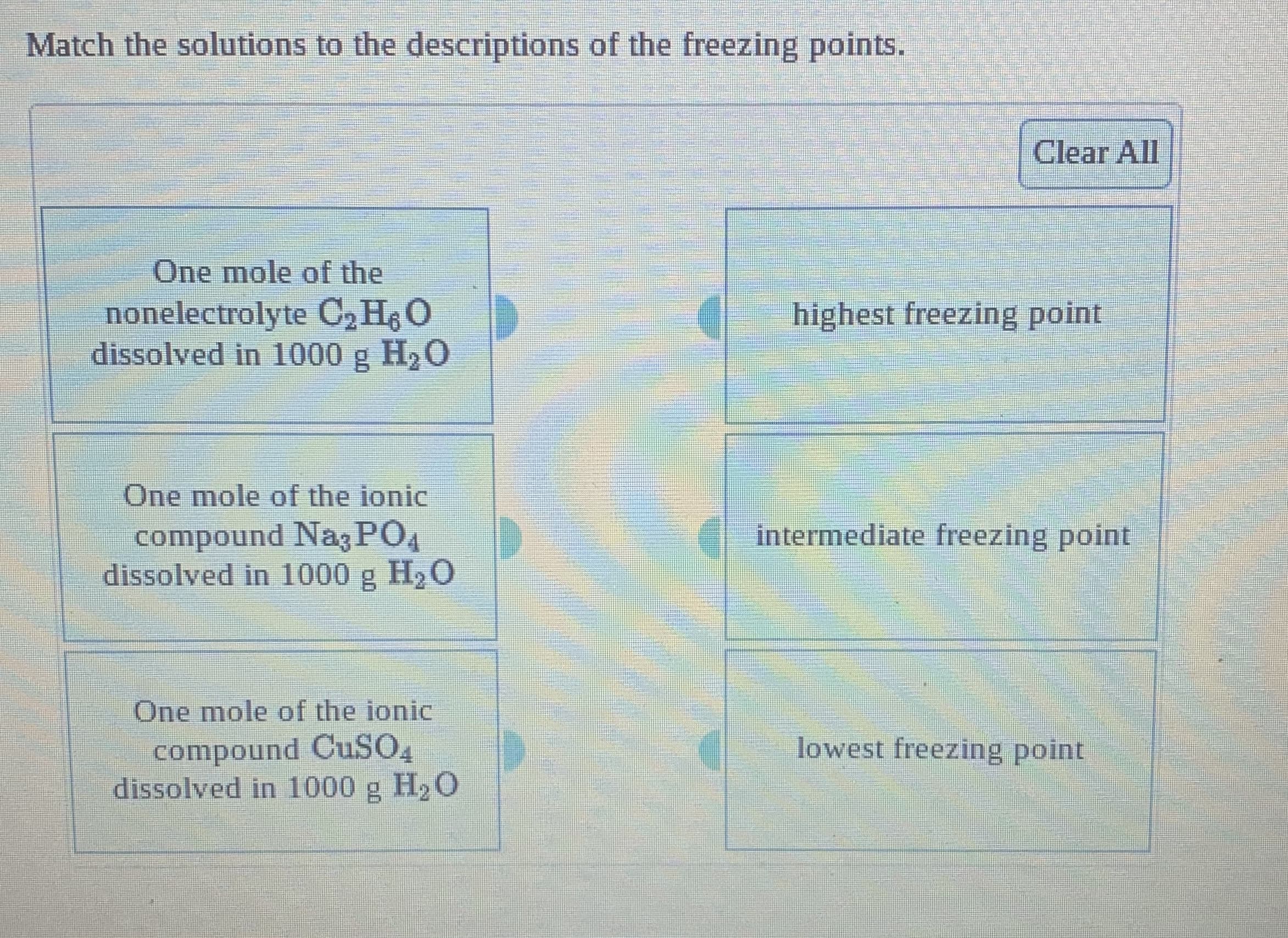
Transcribed Image Text:Match the solutions to the descriptions of the freezing points.
Clear All
One mole of the
nonelectrolyte C, H O
dissolved in 1000 g H,O
highest freezing point
One mole of the ionic
intermediate freezing point
compound Nag PO4
dissolved in 1000 g H2O
One mole of the ionic
compound CUSO,
dissolved in 1000 g H2 O
lowest freezing point
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning