MC1. Grignard reagent and organolithium reagents can be prepared in presence of acidic functional groups. MC2. Gilman reagents react with formaldehyde and gives alcohol. MC3. An S№2 attack on the chiral center would result in inversion of stereochemistry.
MC1. Grignard reagent and organolithium reagents can be prepared in presence of acidic functional groups. MC2. Gilman reagents react with formaldehyde and gives alcohol. MC3. An S№2 attack on the chiral center would result in inversion of stereochemistry.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter24: Carboxylic Acids & Derivatives
Section: Chapter Questions
Problem 26E
Related questions
Question
100%
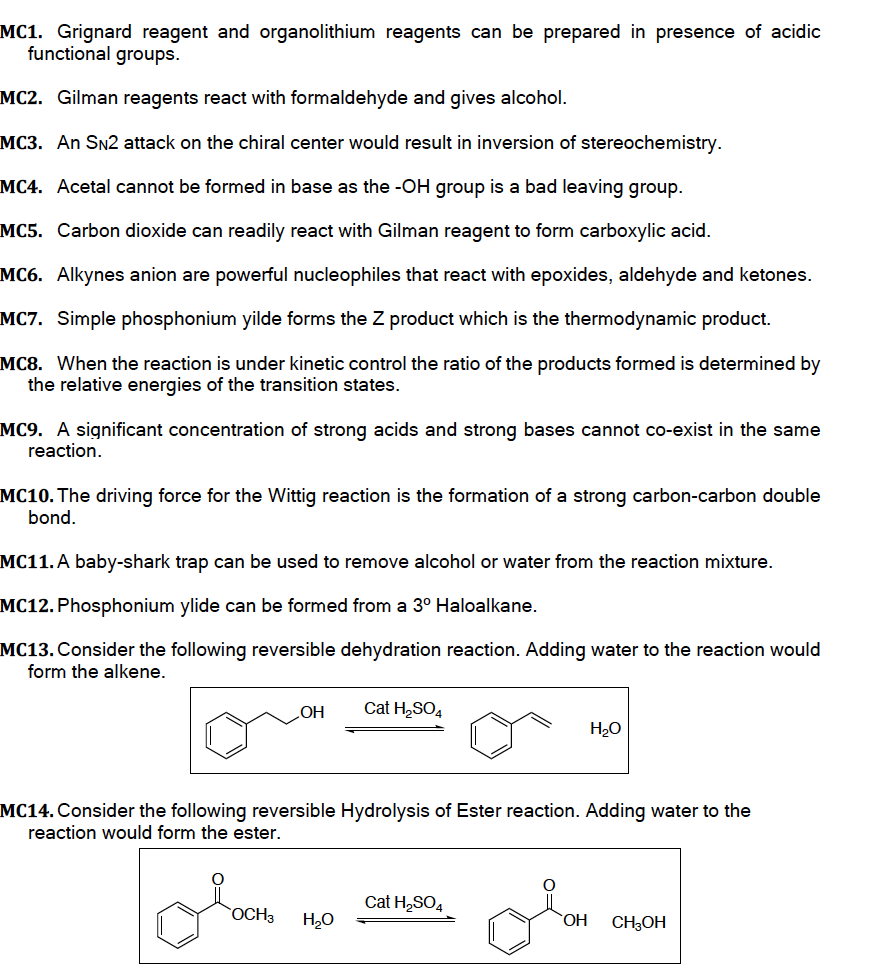
Transcribed Image Text:MC1. Grignard reagent and organolithium reagents can be prepared in presence of acidic
functional groups.
MC2. Gilman reagents react with formaldehyde and gives alcohol.
MC3. An SN2 attack on the chiral center would result in inversion of stereochemistry.
MC4. Acetal cannot be formed in base as the -OH group is a bad leaving group.
MC5. Carbon dioxide can readily react with Gilman reagent to form carboxylic acid.
MC6. Alkynes anion are powerful nucleophiles that react with epoxides, aldehyde and ketones.
MC7. Simple phosphonium yilde forms the Z product which is the thermodynamic product.
MC8. When the reaction is under kinetic control the ratio of the products formed is determined by
the relative energies of the transition states.
MC9. A significant concentration of strong acids and strong bases cannot co-exist in the same
reaction.
MC10. The driving force for the Wittig reaction is the formation of a strong carbon-carbon double
bond.
MC11. A baby-shark trap can be used to remove alcohol or water from the reaction mixture.
MC12. Phosphonium ylide can be formed from a 3º Haloalkane.
MC13. Consider the following reversible dehydration reaction. Adding water to the reaction would
form the alkene.
OH
Cat H₂SO4
OCH3 H₂O
MC14. Consider the following reversible Hydrolysis of Ester reaction. Adding water to the
reaction would form the ester.
H₂O
Cat H₂SO4
OH CH3OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning