MEASUREMENT OF HEAT T01/S08 Calculate the mass (g) of a sample of copper that gained 306.5 J of heat energy when it has a temperature change of 18.1 °C. Specific Heat J/(g °C) 0.384 Cu Molar Heat J/(mol °C) Cu 24.4 the tolerance is +/-2%
MEASUREMENT OF HEAT T01/S08 Calculate the mass (g) of a sample of copper that gained 306.5 J of heat energy when it has a temperature change of 18.1 °C. Specific Heat J/(g °C) 0.384 Cu Molar Heat J/(mol °C) Cu 24.4 the tolerance is +/-2%
Chapter10: Energy
Section: Chapter Questions
Problem 28A
Related questions
Question
Please help me show all the work as I will be using this to study, double and triple check your answers as previous tutors got it wrong
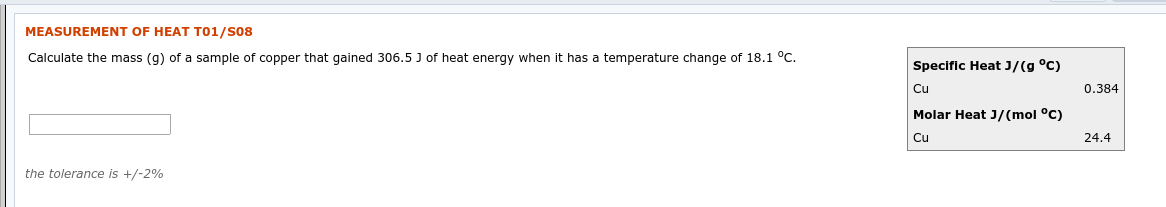
Transcribed Image Text:MEASUREMENT OF HEAT T01/S08
Calculate the mass (g) of a sample of copper that gained 306.5 J of heat energy when it has a temperature change of 18.1 °C.
Specific Heat J/(g °C)
0.384
Cu
Molar Heat J/(mol °C)
Cu
24.4
the tolerance is +/-2%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning