Some polymers may be strengthened considerably by a mechanism called 'branching'. This mechanism can be described as: Select one: O a. Aligning the long chain molecules in closely ordered proximity to each other be elongating the polymer. O b. Attaching molecules of the same mer as very long side groups, by strong covalent bonds to the backbone of the main chain. Oc. Forming covalent double bonds between adjacent molecules by heating in the presence of oxygen or sulphur. Od. Attaching molecules of a different mer as very short side groups, by weak covalent bonds to the backbone of the main chain. The figure below shows a double chain structure of silicon and oxygen. The chemical formula of the chain structure is (SixOyz-)n. What is the value of z for this structure? Select one: О а. -6 Оb. -2 Ос. -5 O d. -8 Ое. -4
Some polymers may be strengthened considerably by a mechanism called 'branching'. This mechanism can be described as: Select one: O a. Aligning the long chain molecules in closely ordered proximity to each other be elongating the polymer. O b. Attaching molecules of the same mer as very long side groups, by strong covalent bonds to the backbone of the main chain. Oc. Forming covalent double bonds between adjacent molecules by heating in the presence of oxygen or sulphur. Od. Attaching molecules of a different mer as very short side groups, by weak covalent bonds to the backbone of the main chain. The figure below shows a double chain structure of silicon and oxygen. The chemical formula of the chain structure is (SixOyz-)n. What is the value of z for this structure? Select one: О а. -6 Оb. -2 Ос. -5 O d. -8 Ое. -4
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 51QAP
Related questions
Question
100%

Transcribed Image Text:Some polymers may be strengthened considerably by a mechanism called 'branching'. This
mechanism can be described as:
Select one:
O a. Aligning the long chain molecules in closely ordered proximity to each other be
elongating the polymer.
O b. Attaching molecules of the same mer as very long side groups, by strong covalent
bonds to the backbone of the main chain.
Oc.
Forming covalent double bonds between adjacent molecules by heating in the
presence of oxygen or sulphur.
Od.
Attaching molecules of a different mer as very short side groups, by weak covalent
bonds to the backbone of the main chain.
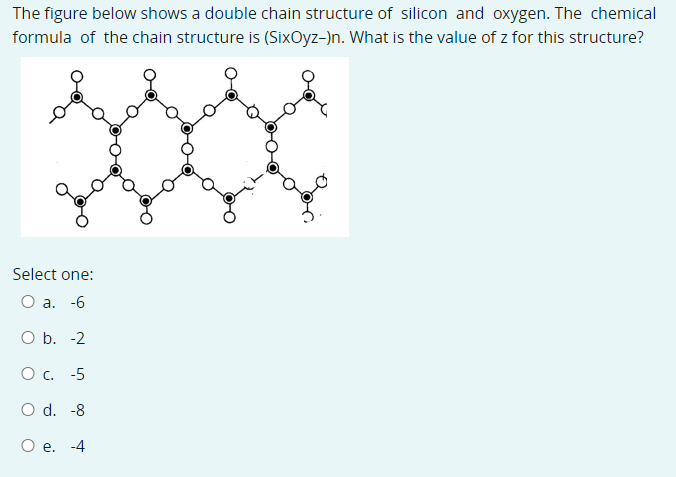
Transcribed Image Text:The figure below shows a double chain structure of silicon and oxygen. The chemical
formula of the chain structure is (SixOyz-)n. What is the value of z for this structure?
Select one:
О а. -6
Оb. -2
Ос. -5
O d. -8
Ое. -4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning