Metal ions in aqueous solutions are solvated, or hydrated, by water molecules. Typically this primary hydration sphere is composed of six water molecules. The hydrated metal ion acts as a weak acid, undergoing a stepwise hydrolysis in which it donates an H* ion from its water ligands to the surrounding free water molecules. Shown is the hydrolysis of Al(H,O)+ (pKa = 4.85) in water. Al(H, O);*(aq) + H,01) = H;0*(aq) + Al(H, O),OH²+(aq) Calculate the pH of a 0.00151 M AICI, solution and determine what fraction of the aluminum is in the form Al(H,O),OH?+ pH = dAI(H, O), (OH)*
Metal ions in aqueous solutions are solvated, or hydrated, by water molecules. Typically this primary hydration sphere is composed of six water molecules. The hydrated metal ion acts as a weak acid, undergoing a stepwise hydrolysis in which it donates an H* ion from its water ligands to the surrounding free water molecules. Shown is the hydrolysis of Al(H,O)+ (pKa = 4.85) in water. Al(H, O);*(aq) + H,01) = H;0*(aq) + Al(H, O),OH²+(aq) Calculate the pH of a 0.00151 M AICI, solution and determine what fraction of the aluminum is in the form Al(H,O),OH?+ pH = dAI(H, O), (OH)*
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.15QAP
Related questions
Question
100%
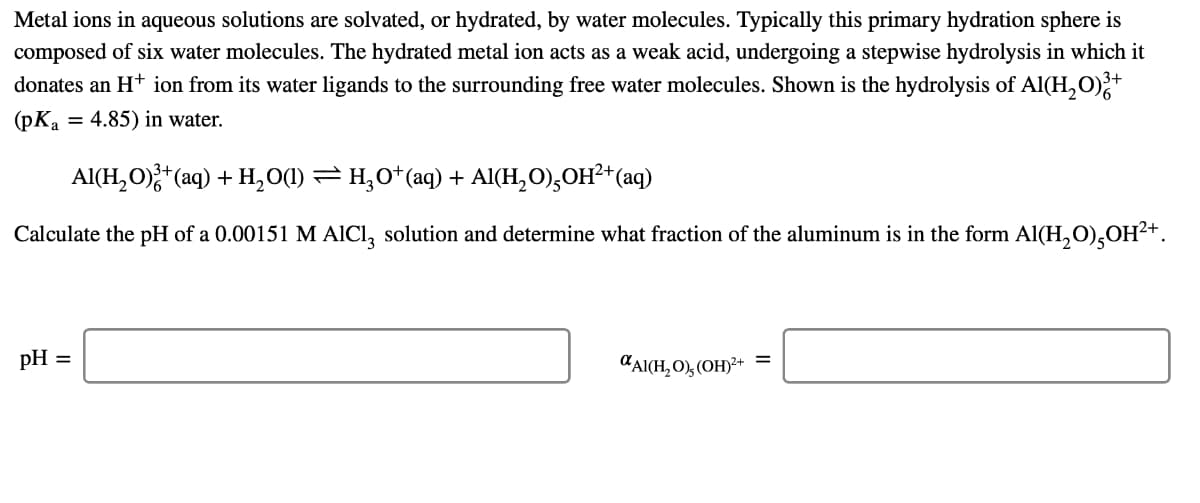
Transcribed Image Text:Metal ions in aqueous solutions are solvated, or hydrated, by water molecules. Typically this primary hydration sphere is
composed of six water molecules. The hydrated metal ion acts as a weak acid, undergoing a stepwise hydrolysis in which it
donates an H* ion from its water ligands to the surrounding free water molecules. Shown is the hydrolysis of Al(H,O);+
(pKa = 4.85) in water.
Al(H, O);*(aq) + H,01) = H;0*(aq) + Al(H, O),OH²+(aq)
Calculate the pH of a 0.00151 M AICI, solution and determine what fraction of the aluminum is in the form Al(H,O),OH²+.
pH =
"AICH,O), (OH)²+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning