Methylamine Ethylamine H3NH₂ C₂H5NH₂ H₂NH3 C₂H5NH3+ 4.38 X 10 5.6 x 10-4 Aniline CH;NH, C6H5NH3 + 3.8 x 10-10 Pyridine C,H,N CH;NH* 1.7 x 10-⁹ 1. Compute the poH of the buffer solution. (Supply answer up to the 1st decimal point)
Methylamine Ethylamine H3NH₂ C₂H5NH₂ H₂NH3 C₂H5NH3+ 4.38 X 10 5.6 x 10-4 Aniline CH;NH, C6H5NH3 + 3.8 x 10-10 Pyridine C,H,N CH;NH* 1.7 x 10-⁹ 1. Compute the poH of the buffer solution. (Supply answer up to the 1st decimal point)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 55P
Related questions
Question
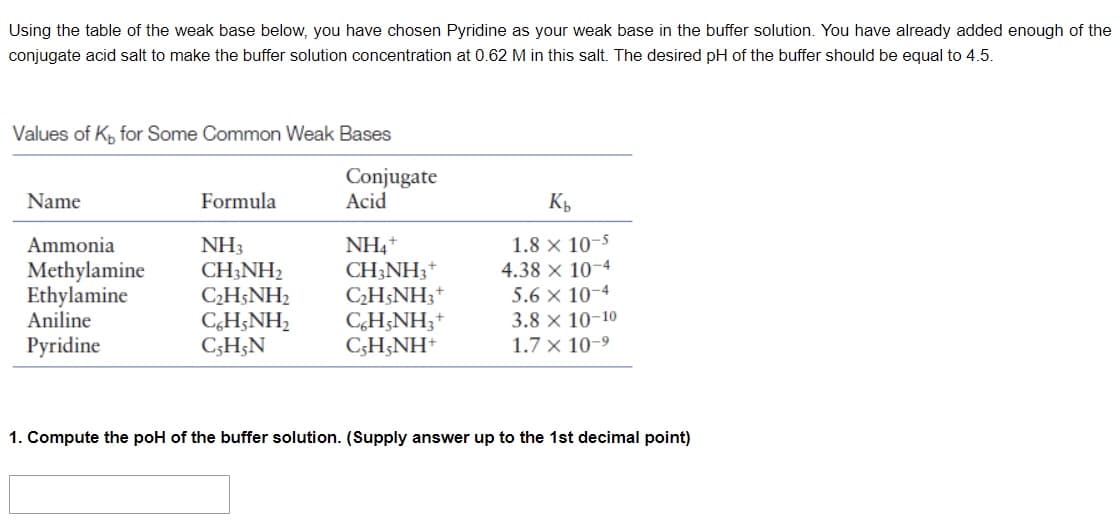
Transcribed Image Text:Using the table of the weak base below, you have chosen Pyridine as your weak base in the buffer solution. You have already added enough of the
conjugate acid salt to make the buffer solution concentration at 0.62 M in this salt. The desired pH of the buffer should be equal to 4.5.
Values of K, for Some Common Weak Bases
Conjugate
Name
Formula
Acid
Kb
Ammonia
NH3
NH4+
1.8 x 10-5
Methylamine CH3NH2
CH3NH3 +
4.38 x 10-4
Ethylamine
C₂H5NH₂
C₂H5NH3+
5.6 x 10-4
Aniline
C6H5NH3 +
3.8 x 10-10
CH;NH,
C,H,N
Pyridine
CH;NH*
1.7 x 10-⁹
1. Compute the poH of the buffer solution. (Supply answer up to the 1st decimal point)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning