Methylhydrazine Chemical Formula CH,N2 Molar Mass 46.07 g/mol Standard boiling point (T°%) AH°vap at Tºb 364 K 40.37 kJ/mol Cliquid at 298.15 K AH°rgas at 298.15 K Molar heat capacity (liquid) Density (liquid) S° (liquid) at 298.15 K ΔΗ, 54.14 kJ/mol 94.50 kJ/mol 134.93 J/mol-K 0.878 g/cm³ 165.94 J/mol K Other thermochemical data at 298.15 K, 1 atm AH°rof CO2«g) AH°rof H2O1) -393.51 kJ/mol -285.83 kJ/mol A. Estimate the standard enthalpy of combustion of methylhydrazine based on the given standard enthalpy of formation values of reactants and products. B. Write the balanced thermochemical equation for the combustion of methylhydrazine. C. Calculate the heat evolved in the combustion of 25.00 liters of methylhydrazine at 298.15 K in air (external pressure of 760 mmHg).
Methylhydrazine Chemical Formula CH,N2 Molar Mass 46.07 g/mol Standard boiling point (T°%) AH°vap at Tºb 364 K 40.37 kJ/mol Cliquid at 298.15 K AH°rgas at 298.15 K Molar heat capacity (liquid) Density (liquid) S° (liquid) at 298.15 K ΔΗ, 54.14 kJ/mol 94.50 kJ/mol 134.93 J/mol-K 0.878 g/cm³ 165.94 J/mol K Other thermochemical data at 298.15 K, 1 atm AH°rof CO2«g) AH°rof H2O1) -393.51 kJ/mol -285.83 kJ/mol A. Estimate the standard enthalpy of combustion of methylhydrazine based on the given standard enthalpy of formation values of reactants and products. B. Write the balanced thermochemical equation for the combustion of methylhydrazine. C. Calculate the heat evolved in the combustion of 25.00 liters of methylhydrazine at 298.15 K in air (external pressure of 760 mmHg).
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 75AP
Related questions
Question
need some help in letters D and E
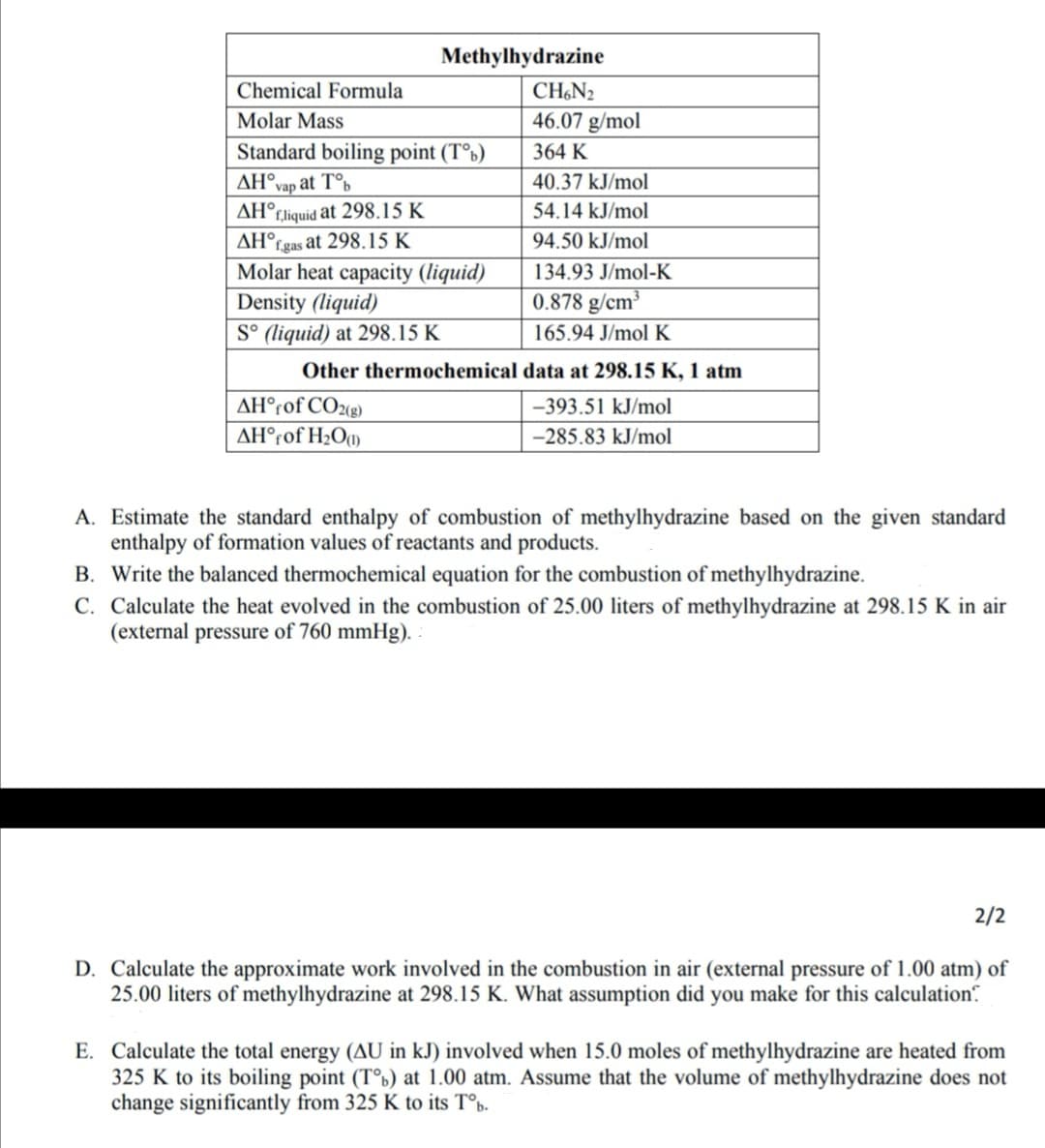
Transcribed Image Text:Methylhydrazine
Chemical Formula
CH,N2
Molar Mass
46.07 g/mol
Standard boiling point (T°b)
AH°vap at T°b
AH°rliquid at 298.15 K
AH°rgas at 298.15 K
Molar heat capacity (liquid)
Density (liquid)
S° (liquid) at 298.15 K
364 K
40.37 kJ/mol
54.14 kJ/mol
94.50 kJ/mol
134.93 J/mol-K
0.878 g/cm³
165.94 J/mol K
Other thermochemical data at 298.15 K, 1 atm
AH°rof CO2(g)
-393.51 kJ/mol
AH°rof H2Ot1)
-285.83 kJ/mol
A. Estimate the standard enthalpy of combustion of methylhydrazine based on the given standard
enthalpy of formation values of reactants and products.
B. Write the balanced thermochemical equation for the combustion of methylhydrazine.
C. Calculate the heat evolved in the combustion of 25.00 liters of methylhydrazine at 298.15 K in air
(external pressure of 760 mmHg). :
2/2
D. Calculate the approximate work involved in the combustion in air (external pressure of 1.00 atm) of
25.00 liters of methylhydrazine at 298.15 K. What assumption did you make for this calculation.
E. Calculate the total energy (AU in kJ) involved when 15.0 moles of methylhydrazine are heated from
325 K to its boiling point (T°%) at 1.00 atm. Assume that the volume of methylhydrazine does not
change significantly from 325 K to its T°p.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning