midnight). Part IA: Answer the following questions in your own words. 1. What does a large equilibrium constant tell us about a reaction? What about a small one? 2. A solid is a component of an equilibrium equation. Does it have any impact on the reaction at all? Does it have an impact on the equilibrium?
midnight). Part IA: Answer the following questions in your own words. 1. What does a large equilibrium constant tell us about a reaction? What about a small one? 2. A solid is a component of an equilibrium equation. Does it have any impact on the reaction at all? Does it have an impact on the equilibrium?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter13: Chemical Equilibrium
Section: Chapter Questions
Problem 21Q: For a typical equilibrium problem, the value of K and the initial reaction conditions are given for...
Related questions
Question
Need help with question 1 and 2
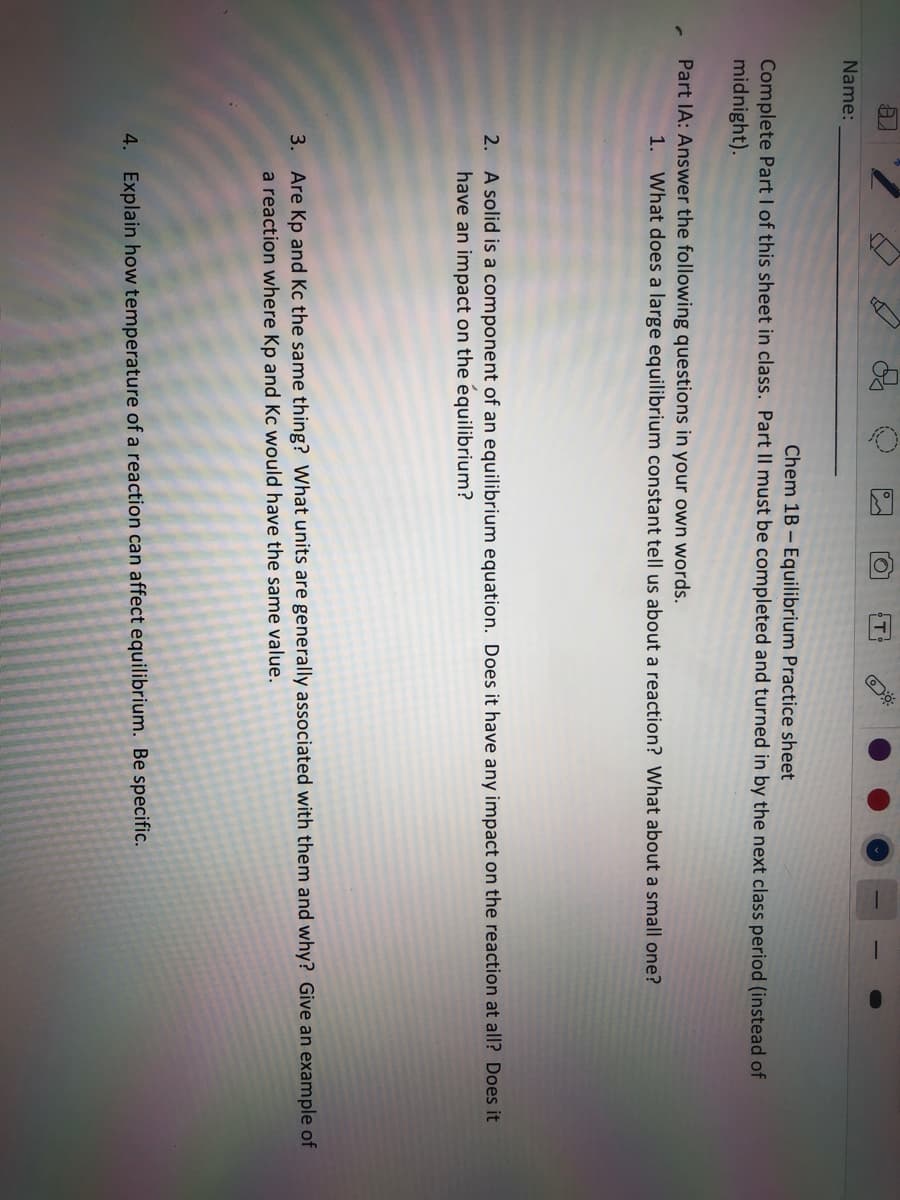
Transcribed Image Text:Name:
Chem 1B - Equilibrium Practice sheet
Complete Part I of this sheet in class. Part Il must be completed and turned in by the next class period (instead of
midnight).
Part IA: Answer the following questions in your own words.
1. What does a large equilibrium constant tell us about a reaction? What about a small one?
2. A solid is a component of an equilibrium equation. Does it have any impact on the reaction at all? Does it
have an impact on the equilibrium?
3. Are Kp and Kc the same thing? What units are generally associated with them and why? Give an example of
a reaction where Kp and Kc would have the same value.
4. Explain how temperature of a reaction can affect equilibrium. Be specific.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning