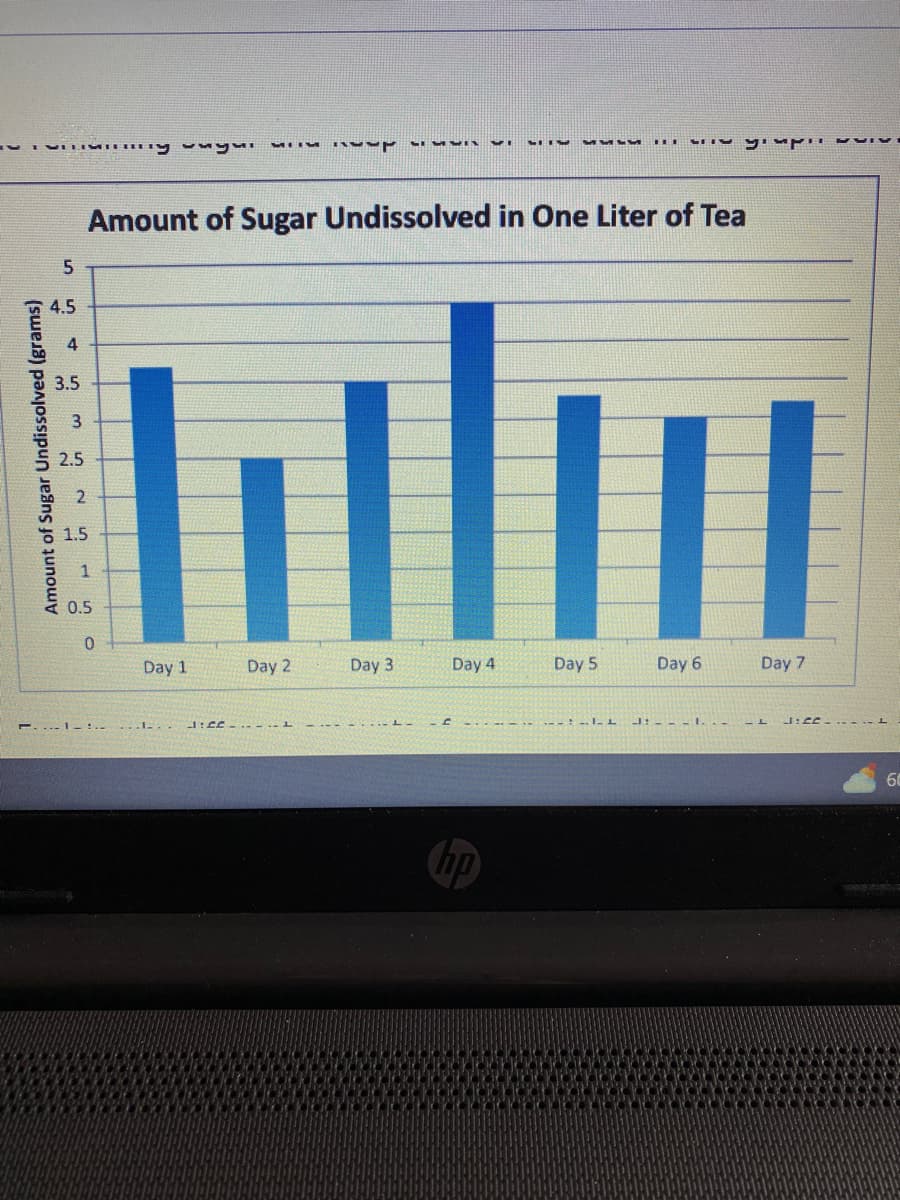
might dissolve at different times. Consider: • which day the least sugar dissolve in which day the most sugar dissolved  • what kind of cause less sugar to dissolve on some days • what does student could do to his drink to make more sugar dissolve. be sure to consider the completeness of your response, supporting
might dissolve at different times. Consider: • which day the least sugar dissolve in which day the most sugar dissolved  • what kind of cause less sugar to dissolve on some days • what does student could do to his drink to make more sugar dissolve. be sure to consider the completeness of your response, supporting
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.10QAP
Related questions
Question
Explain why different amounts of sugar might dissolve at different times.
Consider:
• which day the least sugar dissolve in which day the most sugar dissolved 
• what kind of cause less sugar to dissolve on some days
• what does student could do to his drink to make more sugar dissolve.
be sure to consider the completeness of your response, supporting details, and accurate use of terms. Your response should be 6 to 8 complete sentences. 
Transcribed Image Text:Amount of Sugar Undissolved (grams)
5
4.5
4
in s
3.5
3
2.5
2
1.5
1
0.5
0
y unyu
-...1-1._
י -
Amount of Sugar Undissolved in One Liter of Tea
Day 1
..I..
1: CC-
Day 2
SEMMIN
Day 3
F
VI
Day 4
hp
SETY MMS HE
1 YILIN
II
Day 6
Day 5
---: . 1. L
J:--- I...
Day 7
-L J:CE....L
60
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,



Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT