mol- By process, 560 g of CH1206 (180.2) is broken down with 1200 g of O2 (16-) O2 co, + H,0 а. Which of the two reactants is the limiting reagent? b. Calculate the mass of co, formed.
mol- By process, 560 g of CH1206 (180.2) is broken down with 1200 g of O2 (16-) O2 co, + H,0 а. Which of the two reactants is the limiting reagent? b. Calculate the mass of co, formed.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter6: Properties Of Hydrates
Section: Chapter Questions
Problem 1ASA: A student is given a sample of a pink manganese (II) chloride hydrate. She weighs the sample in a...
Related questions
Question
Transcribed Image Text:ARUCATION
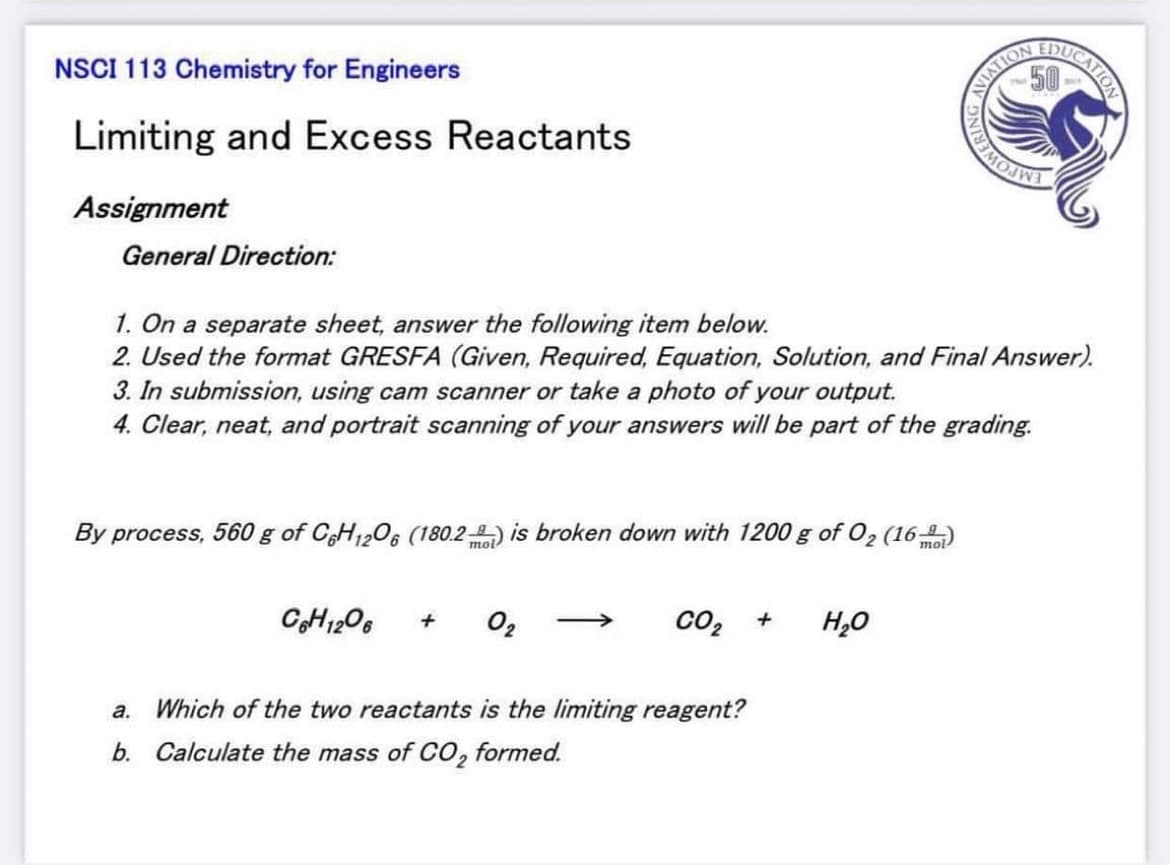
NSCI 113 Chemistry for Engineers
Limiting and Excess Reactants
Assignment
General Direction:
1. On a separate sheet, answer the following item below.
2. Used the format GRESFA (Given, Required, Equation, Solution, and Final Answer).
3. In submission, using cam scanner or take a photo of your output.
4. Clear, neat, and portrait scanning of your answers will be part of the grading.
By process, 560 g of CH1206 (180.2-) is broken down with 1200 g of O2 (16-)
mol
CH1206
O2
co2
H,0
a. Which of the two reactants is the limiting reagent?
b. Calculate the mass of CO, formed.
MATION
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT