2 H2(g) + O2(g) → 2 H2O(1) 3. Calculate: 2He/10z=2,2:1 a. the stoichiometric ratio of moles H2 to moles O2 b. the actual moles H2 to moles O2 when 3.50 mol H2 is mixed with 2.00 mol O2 c. the limiting reactant (H2 or O2) for the mixture in part (b) d. the theoretical yield, in moles, of H20 for the mixture in part (b)
2 H2(g) + O2(g) → 2 H2O(1) 3. Calculate: 2He/10z=2,2:1 a. the stoichiometric ratio of moles H2 to moles O2 b. the actual moles H2 to moles O2 when 3.50 mol H2 is mixed with 2.00 mol O2 c. the limiting reactant (H2 or O2) for the mixture in part (b) d. the theoretical yield, in moles, of H20 for the mixture in part (b)
Chapter13: Isolation Of Eugenol From Clov
Section: Chapter Questions
Problem 9Q
Related questions
Question
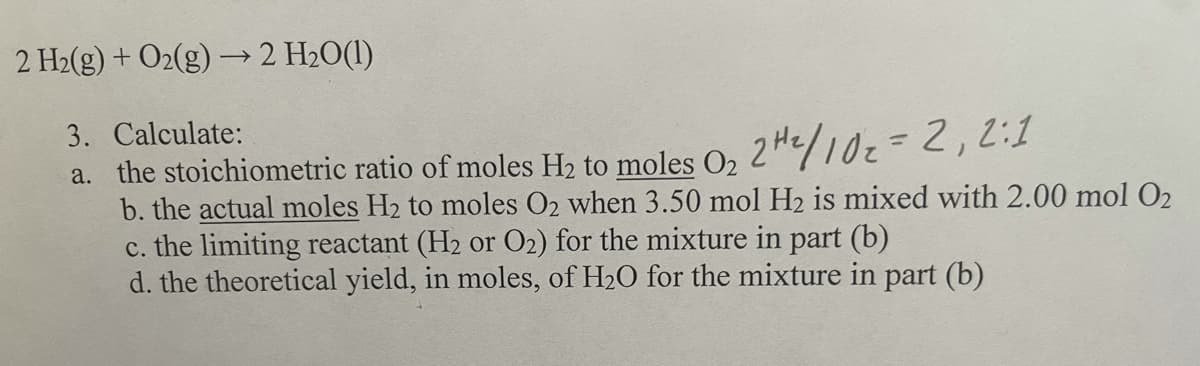
Transcribed Image Text:2 H2(g) + O2(g) –→2 H2O(1)
3. Calculate:
a. the stoichiometric ratio of moles H2 to moles O, 2H/102=2,2:1
b. the actual moles H2 to moles O2 when 3.50 mol H2 is mixed with 2.00 mol O2
c. the limiting reactant (H2 or O2) for the mixture in part (b)
d. the theoretical yield, in moles, of H2O for the mixture in part (b)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning