Part C A sample of propane, C3 H8, contains 15.8 moles of carbon atoms. How many total moles of atoms does the sample contain? Express the total number of moles of carbon and hydrogen numerically. View Available Hint(s) VAD ? moles Submit Provide Feedback Next> P Pearson cation Inc. All rights reserved. | Terms of Use Privacy Policy I Permissions | Contact Us II F8 F9 F11 F6 F7 F10 F12 ) & 9 7 8 1 delete { U P enter K
Part C A sample of propane, C3 H8, contains 15.8 moles of carbon atoms. How many total moles of atoms does the sample contain? Express the total number of moles of carbon and hydrogen numerically. View Available Hint(s) VAD ? moles Submit Provide Feedback Next> P Pearson cation Inc. All rights reserved. | Terms of Use Privacy Policy I Permissions | Contact Us II F8 F9 F11 F6 F7 F10 F12 ) & 9 7 8 1 delete { U P enter K
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 21ALQ: True or false? The atom with the largest subscript in a formula is the atom with the largest percent...
Related questions
Question
100%
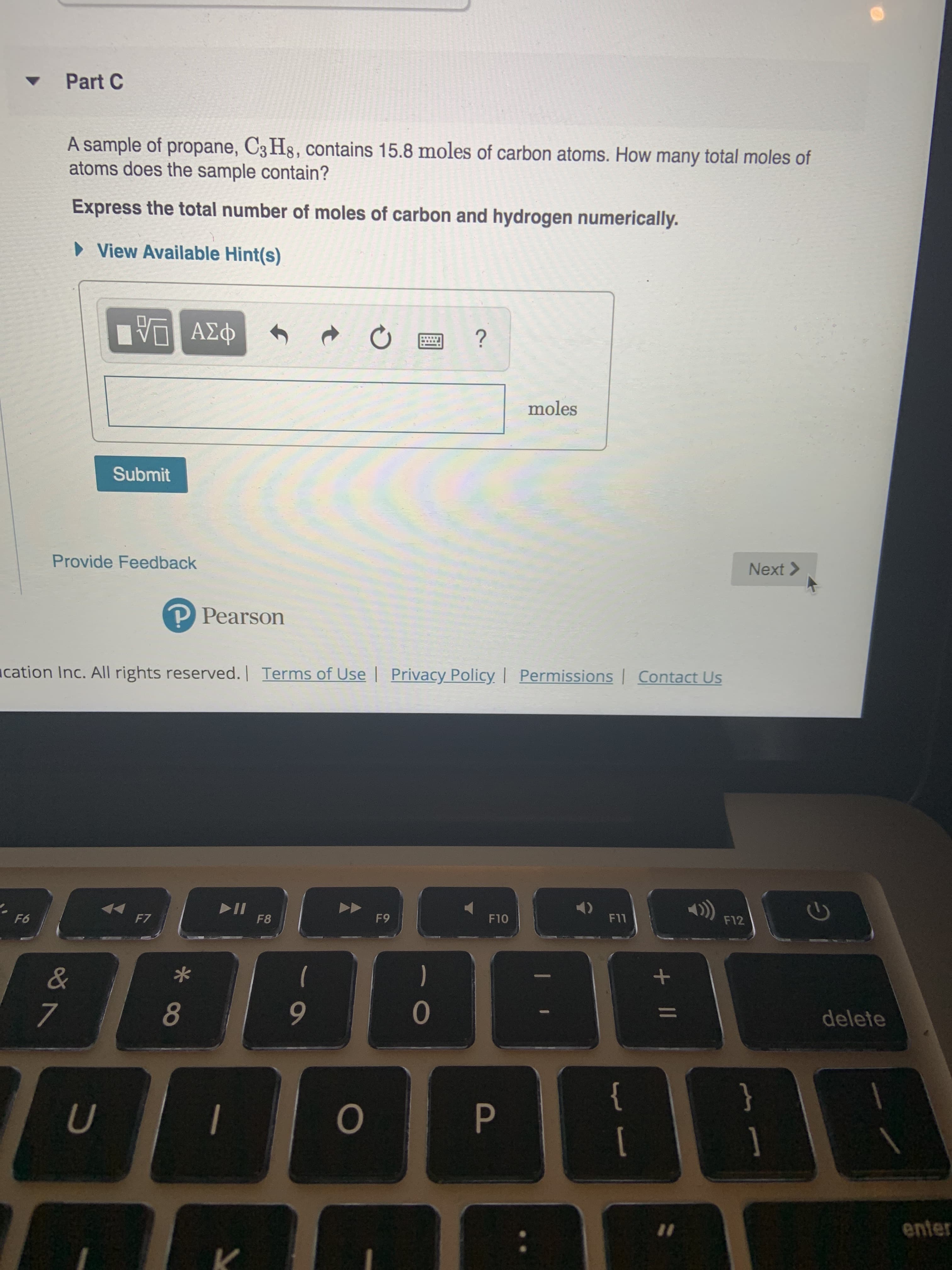
Transcribed Image Text:Part C
A sample of propane, C3 H8, contains 15.8 moles of carbon atoms. How many total moles of
atoms does the sample contain?
Express the total number of moles of carbon and hydrogen numerically.
View Available Hint(s)
VAD
?
moles
Submit
Provide Feedback
Next>
P Pearson
cation Inc. All rights reserved. | Terms of Use
Privacy Policy I Permissions |
Contact Us
II
F8
F9
F11
F6
F7
F10
F12
)
&
9
7
8
1
delete
{
U
P
enter
K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
