Molecular Models: Lewis Electron Dot Structures and Molecular Geometry Part A: Use the molecular model kit and complete the following table. Total Sketch of Molecular number of Lewis Name of the Molecule is three- electron dot molecular polar or попрolar? formula valence dimensional structure electrons geometry geometry 5+5 = 10 NEN Non polat Trigonal Polar Pyramidal N2 NEN Linear %3D 5+311 HNHH NH3 H2S 2メ1+G CO2 PF3 CH4 SO2 HCN H2CO
Molecular Models: Lewis Electron Dot Structures and Molecular Geometry Part A: Use the molecular model kit and complete the following table. Total Sketch of Molecular number of Lewis Name of the Molecule is three- electron dot molecular polar or попрolar? formula valence dimensional structure electrons geometry geometry 5+5 = 10 NEN Non polat Trigonal Polar Pyramidal N2 NEN Linear %3D 5+311 HNHH NH3 H2S 2メ1+G CO2 PF3 CH4 SO2 HCN H2CO
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.89QE
Related questions
Question
100%
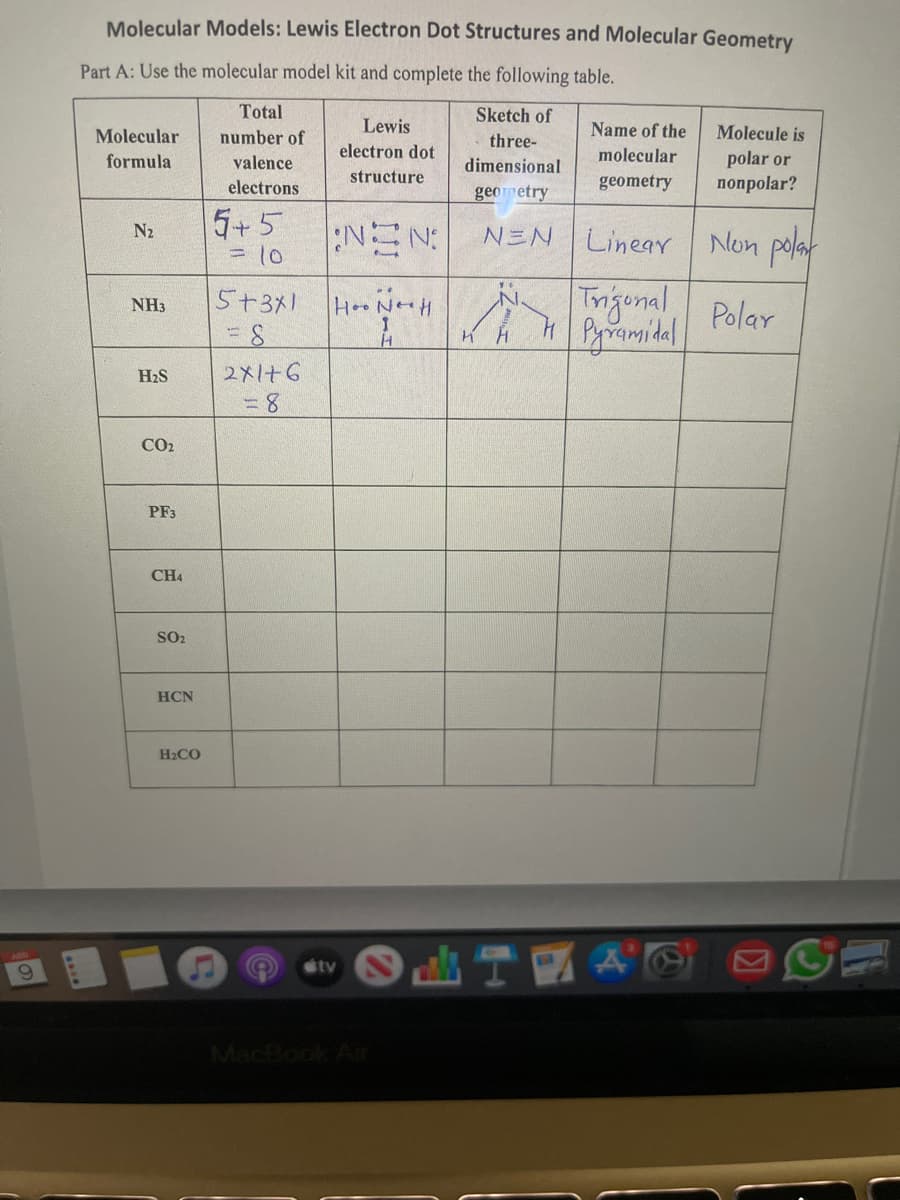
Transcribed Image Text:Molecular Models: Lewis Electron Dot Structures and Molecular Geometry
Part A: Use the molecular model kit and complete the following table.
Total
Sketch of
Molecular
number of
Lewis
Name of the
Molecule is
three-
electron dot
molecular
polar or
пoпрolar?
formula
valence
dimensional
electrons
structure
geometry
geometry
5+5
NEN:
= 10
Linear Non polat
N2
NEN
Tngenal Polar
5+311
HNe H
NH3
H2S
2メ+G
CO2
PF3
CH4
SO2
HCN
H2CO
tv
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 14 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning