Molecules of four imaginary substances are sketched in the table below. Each sketch is shaded to show the electrostatic potential at the surface of the molecule. Rank these substances in decreasing order of the strength of the intermolecular forces in them. In other words, choose 1 next to the substance in which the molecules exert the strongest intermolecular forces on each other. Choose 2 next to the substance in which the molecules exert the second strongest intermolecular forces on each other, and so forth. Note: all of the molecules are neutral, and you may assume none of them experience hydrogen bonding. electrostatic strength of intermolecular force substance potential map (Choose one) B (Choose one) v (Choose one) (Choose one) v
Molecules of four imaginary substances are sketched in the table below. Each sketch is shaded to show the electrostatic potential at the surface of the molecule. Rank these substances in decreasing order of the strength of the intermolecular forces in them. In other words, choose 1 next to the substance in which the molecules exert the strongest intermolecular forces on each other. Choose 2 next to the substance in which the molecules exert the second strongest intermolecular forces on each other, and so forth. Note: all of the molecules are neutral, and you may assume none of them experience hydrogen bonding. electrostatic strength of intermolecular force substance potential map (Choose one) B (Choose one) v (Choose one) (Choose one) v
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 1RQ: What are intermolecular forces? How do they differ from intramolecular forces? What are...
Related questions
Question
100%
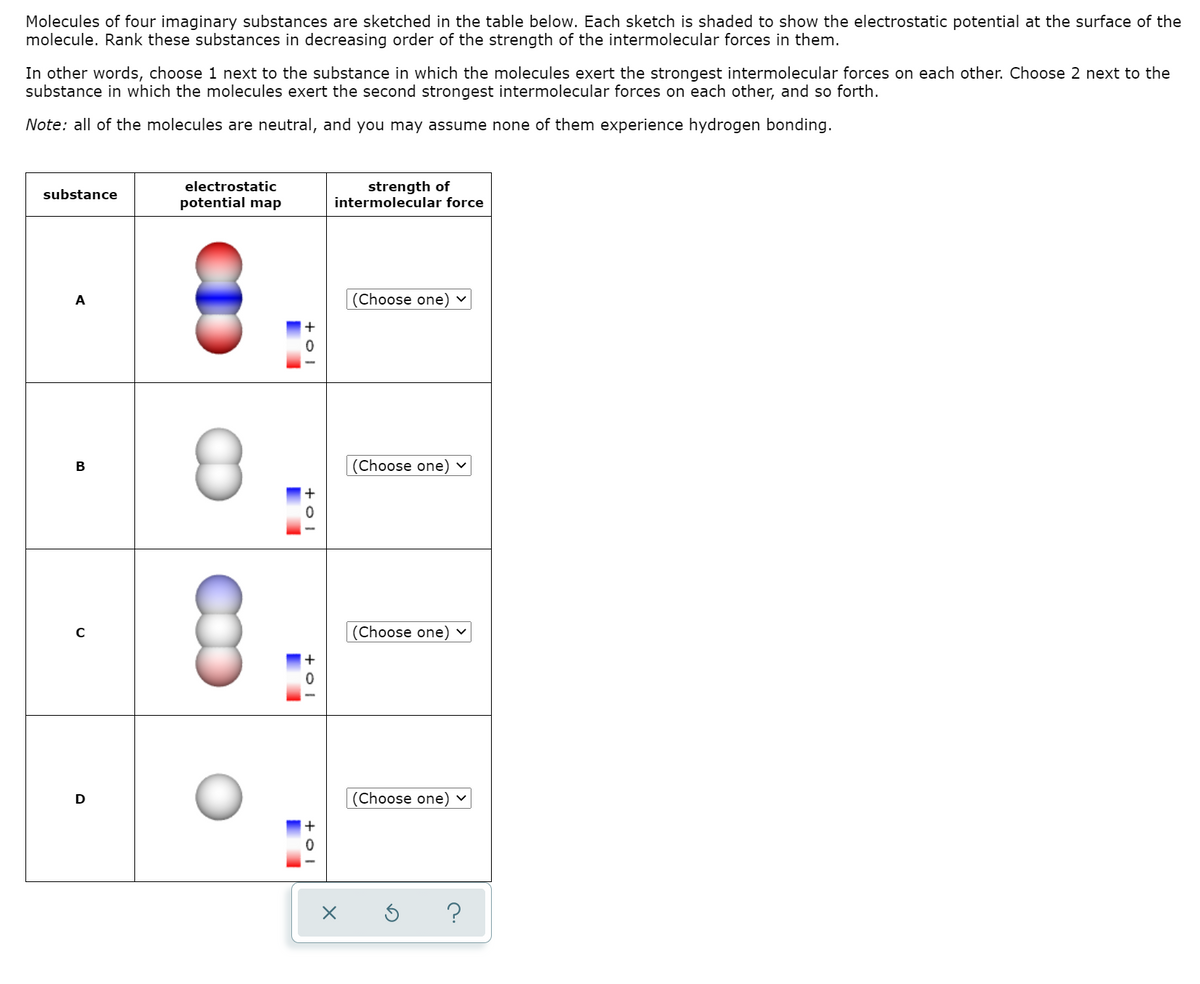
Transcribed Image Text:Molecules of four imaginary substances are sketched in the table below. Each sketch is shaded to show the electrostatic potential at the surface of the
molecule. Rank these substances in decreasing order of the strength of the intermolecular forces in them.
In other words, choose 1 next to the substance in which the molecules exert the strongest intermolecular forces on each other. Choose 2 next to the
substance in which the molecules exert the second strongest intermolecular forces on each other, and so forth.
Note: all of the molecules are neutral, and you may assume none of them experience hydrogen bonding.
strength of
intermolecular force
electrostatic
substance
potential map
A
(Choose one) ▼
+
B
|(Choose one) ♥
(Choose one)
D
Choose one)
+ O I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning