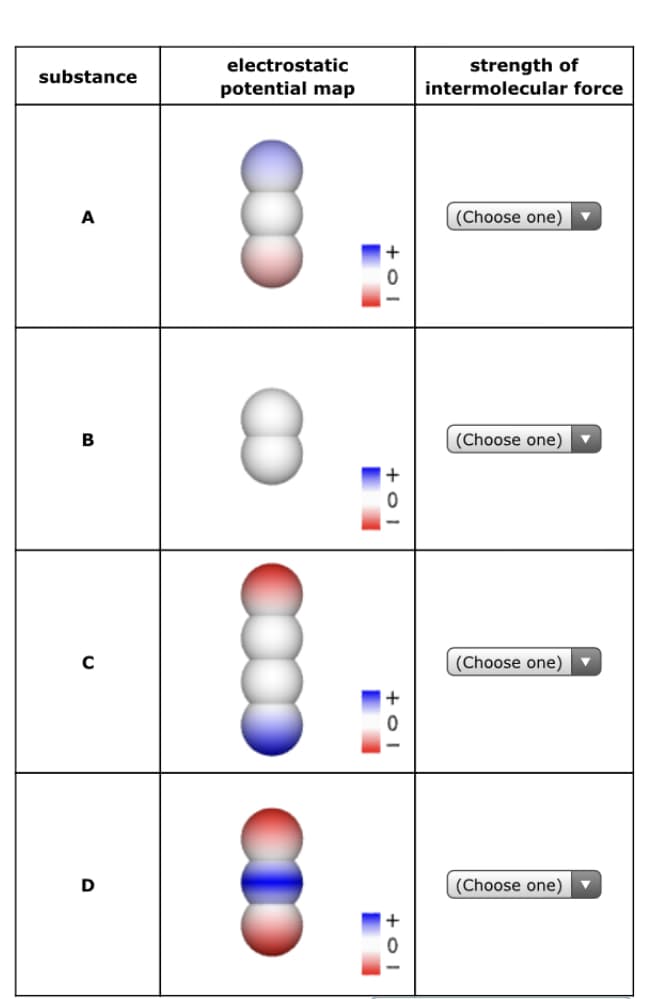
Molecules of four imaginary substances are sketched in the table below. Each sketch is shaded to show the electrostatic potential at the surface of the molecule. Rank these substances in decreasing order of the strength of the intermolecular forces in them. In other words, choose 1 next to the substance in which the molecules exert the strongest intermolecular forces on each other. Choose 2 next to the substance in which the molecules exert the second strongest intermolecular forces on each other, and so forth. Note: all of the molecules are neutral, and you may assume none of them experience hydrogen bonding.
Molecules of four imaginary substances are sketched in the table below. Each sketch is shaded to show the electrostatic potential at the surface of the molecule. Rank these substances in decreasing order of the strength of the intermolecular forces in them. In other words, choose 1 next to the substance in which the molecules exert the strongest intermolecular forces on each other. Choose 2 next to the substance in which the molecules exert the second strongest intermolecular forces on each other, and so forth. Note: all of the molecules are neutral, and you may assume none of them experience hydrogen bonding.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter13: Introduction To Symmetry In Quantum Mechanics
Section: Chapter Questions
Problem 13.40E: Explain why a molecule with a center of inversion cannot have a dipole moment.
Related questions
Question
Please help me out.

Transcribed Image Text:Molecules of four imaginary substances are sketched in the table below. Each sketch is
shaded to show the electrostatic potential at the surface of the molecule. Rank these
substances in decreasing order of the strength of the intermolecular forces in them.
In other words, choose 1 next to the substance in which the molecules exert the strongest
intermolecular forces on each other. Choose 2 next to the substance in which the molecules
exert the second strongest intermolecular forces on each other, and so forth.
Note: all of the molecules are neutral, and you may assume none of them experience
hydrogen bonding.
Transcribed Image Text:strength of
intermolecular force
electrostatic
substance
potential map
A
(Choose one)
В
(Choose one)
(Choose one)
(Choose one)
+ o |
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,