Moles In this experiment you will use grains of rice as an analogy for atoms and experience why chemists find moles useful as a unit. How many grains of rice fit in a 50 mL beaker? You probably don't want to count them all, just as chemists don't want to count atoms-but if we had a relationship between mass and the number of grains of rice, we could easily calculate the answer. How many grains do you guess would fit in the 50 mL beaker? \.500 Now, start by determining the average mass of a single grain of rice: Count out 10, 30, and 50 grains of rice and use a weigh boat to determine the total weight of the rice. Trial 1 Trial 2 Trial 3 10 30 50 Number of grains of псе: boat: 0.Co 323 0.c6323/0.16323 grains: 0.81041.1702 rice grains: 0.7810.53790.8966 Mass of empty 1.5289 Mass of boat + rice *Mass of only the *Mass per single grain of rice: O, O 6210.01620.0162 1. Which value for the mass per grain of rice do you think is the most reliable, and what makes it the best? 2. Use your most reliable value for the mass per grain of rice to determine the Gross Mass of the rice: the mass of a gross (a dozen dozen, or 144) of rice grains. Your unit should be grams/gross. grams/gross
Moles In this experiment you will use grains of rice as an analogy for atoms and experience why chemists find moles useful as a unit. How many grains of rice fit in a 50 mL beaker? You probably don't want to count them all, just as chemists don't want to count atoms-but if we had a relationship between mass and the number of grains of rice, we could easily calculate the answer. How many grains do you guess would fit in the 50 mL beaker? \.500 Now, start by determining the average mass of a single grain of rice: Count out 10, 30, and 50 grains of rice and use a weigh boat to determine the total weight of the rice. Trial 1 Trial 2 Trial 3 10 30 50 Number of grains of псе: boat: 0.Co 323 0.c6323/0.16323 grains: 0.81041.1702 rice grains: 0.7810.53790.8966 Mass of empty 1.5289 Mass of boat + rice *Mass of only the *Mass per single grain of rice: O, O 6210.01620.0162 1. Which value for the mass per grain of rice do you think is the most reliable, and what makes it the best? 2. Use your most reliable value for the mass per grain of rice to determine the Gross Mass of the rice: the mass of a gross (a dozen dozen, or 144) of rice grains. Your unit should be grams/gross. grams/gross
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter4: Determination Of A Chemical Formula
Section: Chapter Questions
Problem 2ASA: If one can find the ratio of the number of moles of the elements in a compound to one another, one...
Related questions
Question
Transcribed Image Text:Namé:
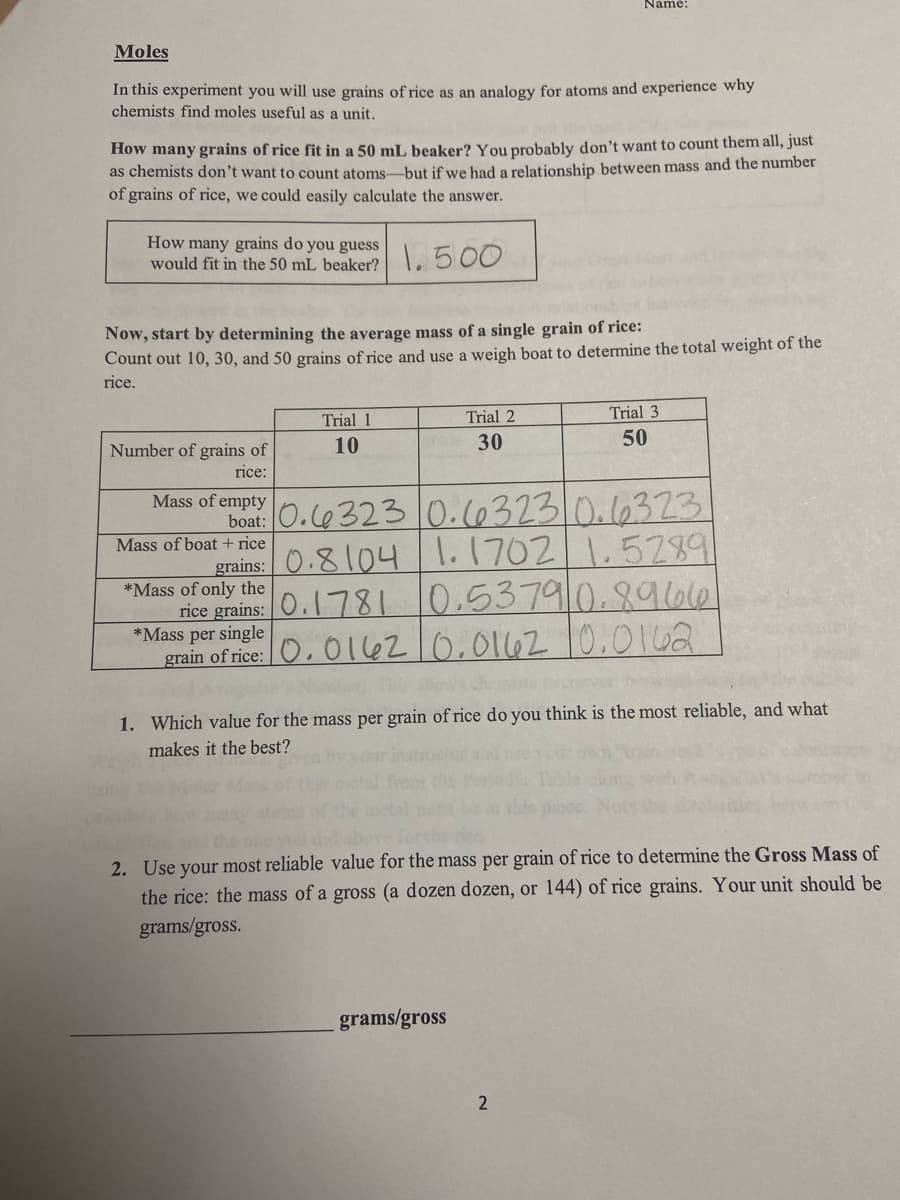
Moles
In this experiment you will use grains of rice as an analogy for atoms and experience why
chemists find moles useful as a unit.
How many grains of rice fit in a 50 mL beaker? You probably don't want to count them all, just
as chemists don't want to count atoms-but if we had a relationship between mass and the number
of grains of rice, we could easily calculate the answer.
How many grains do you guess
would fit in the 50 mL beaker?
1.500
Now, start by determining the average mass of a single grain of rice:
Count out 10, 30, and 50 grains of rice and use a weigh boat to determine the total weight of the
rice.
Trial 1
Trial 2
Trial 3
Number of grains of
10
30
50
rice:
boat: 0. Ce 323 0.c6323.0.16323
grains: 0.81041.1702
rice grains: O.781 10.5379 0.8966
Mass of empty
1.5289
Mass of boat + rice
*Mass of only the
*Mass per single
grain of rice: O, O 1l6210,0162 10.0162
1. Which value for the mass per grain of rice do you think is the most reliable, and what
makes it the best?
2. Use your most reliable value for the mass per grain of rice to determine the Gross Mass of
the rice: the mass of a gross (a dozen dozen, or 144) of rice grains. Your unit should be
grams/gross.
grams/gross
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning