Motif Metal atom Metal atom Metal atom Crystal lattice Primitive cubic lattice Face-centered Body-centered cubic lattice cubic lattice Crystal structure (a) Primitive (b) Body-centered cubic metal (c) Face-centered (d) Corner atoms are cubic metal cubic metal shared by eight unit cells AFigure 12.11 .. ---- ---Lin hlL-
Motif Metal atom Metal atom Metal atom Crystal lattice Primitive cubic lattice Face-centered Body-centered cubic lattice cubic lattice Crystal structure (a) Primitive (b) Body-centered cubic metal (c) Face-centered (d) Corner atoms are cubic metal cubic metal shared by eight unit cells AFigure 12.11 .. ---- ---Lin hlL-
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter21: The Solid State: Crystals
Section: Chapter Questions
Problem 21.31E
Related questions
Question
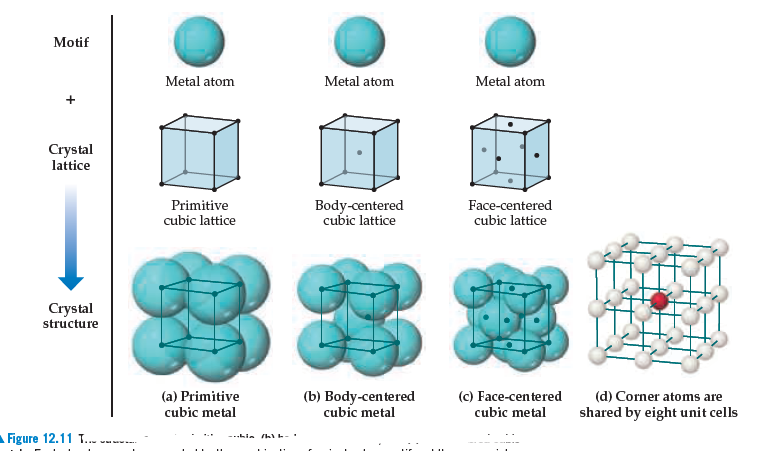
Is it possible for all atoms in an ionic compound to lie on the lattice points as
they do in the metallic structures shown in Figure 12.11?
Transcribed Image Text:Motif
Metal atom
Metal atom
Metal atom
Crystal
lattice
Primitive
cubic lattice
Face-centered
Body-centered
cubic lattice
cubic lattice
Crystal
structure
(a) Primitive
(b) Body-centered
cubic metal
(c) Face-centered
(d) Corner atoms are
cubic metal
cubic metal
shared by eight unit cells
AFigure 12.11 .. ----
---Lin hlL-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning