Moving to another question will save this response. Question 2 What is the net ionic equation for the reaction of aqueous potassium hydroxide and aqueous magnesium chlori 1S K (aq) + OH (aq) KOH(s) КОНЕ) K*(aq) + Cl (aq) →KCI(s) Mg"(aq) + 2 CF (aq) MgCl2(s) 2+ o Mg-(aq)+3 OH (aq)Mg(0H)3(s) Mg-(aq) -2 OH (aq) Mg(OH)2(s) Me (aq) - Он (аq) — MgОН (9) MGOH (s A Moving to another question will save this response. search hp f11 f12 fg f10 f6 f7 f8 f4 f5 I미 & %24 4. 6. 7. 80 T. | 0O
Moving to another question will save this response. Question 2 What is the net ionic equation for the reaction of aqueous potassium hydroxide and aqueous magnesium chlori 1S K (aq) + OH (aq) KOH(s) КОНЕ) K*(aq) + Cl (aq) →KCI(s) Mg"(aq) + 2 CF (aq) MgCl2(s) 2+ o Mg-(aq)+3 OH (aq)Mg(0H)3(s) Mg-(aq) -2 OH (aq) Mg(OH)2(s) Me (aq) - Он (аq) — MgОН (9) MGOH (s A Moving to another question will save this response. search hp f11 f12 fg f10 f6 f7 f8 f4 f5 I미 & %24 4. 6. 7. 80 T. | 0O
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 143QRT: The present average concentration (mass percent) of magnesium ions in seawater is 0.13%. A chemistry...
Related questions
Question
Transcribed Image Text:Moving to another question will save this response.
Question 2
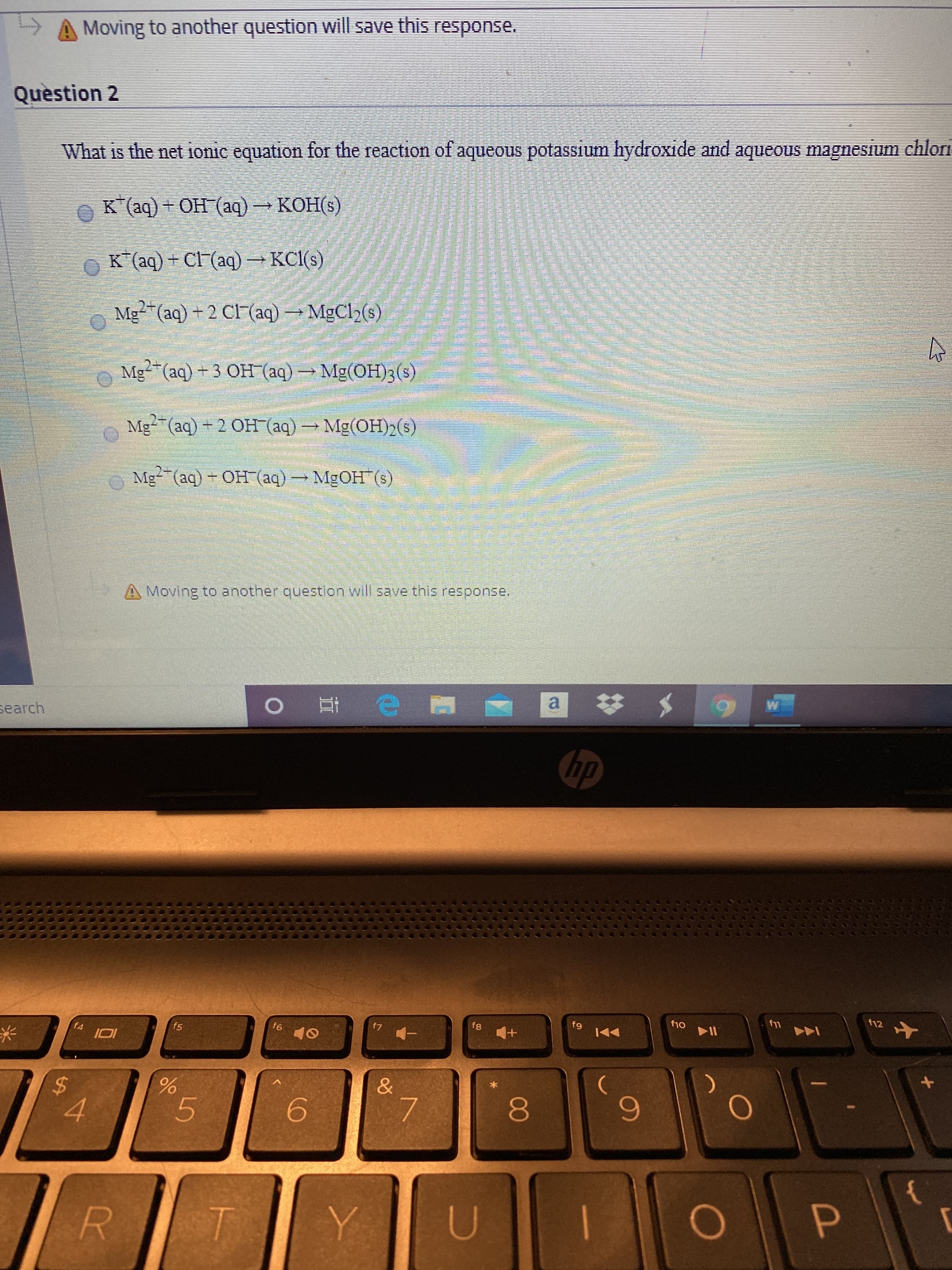
What is the net ionic equation for the reaction of aqueous potassium hydroxide and aqueous magnesium chlori
1S
K (aq) + OH (aq) KOH(s)
КОНЕ)
K*(aq) + Cl (aq) →KCI(s)
Mg"(aq) + 2 CF (aq) MgCl2(s)
2+
o Mg-(aq)+3 OH (aq)Mg(0H)3(s)
Mg-(aq) -2 OH (aq) Mg(OH)2(s)
Me (aq) - Он (аq) — MgОН (9)
MGOH (s
A Moving to another question will save this response.
search
hp
f11
f12
fg
f10
f6
f7
f8
f4
f5
I미
&
%24
4.
6.
7.
80
T.
|
0O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning