0.172 g of potassium hydrogen phthalate (KHP) was titrated with NaOH. When 31.5 mL of the KOH had been added to the acid, the indicator (phenolphthalein), changed color. What is the concentration of the NaOH solution? The molar mass of KHP is 204 g/mol. Enter your answer with three decimal places. Example: 0.544 Do not include units. 0.027
0.172 g of potassium hydrogen phthalate (KHP) was titrated with NaOH. When 31.5 mL of the KOH had been added to the acid, the indicator (phenolphthalein), changed color. What is the concentration of the NaOH solution? The molar mass of KHP is 204 g/mol. Enter your answer with three decimal places. Example: 0.544 Do not include units. 0.027
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 126AP: You pour 150.0 mL of a 0.250 M lead(ll) nitrate solution into an empty 500-mL flask. What is the...
Related questions
Question
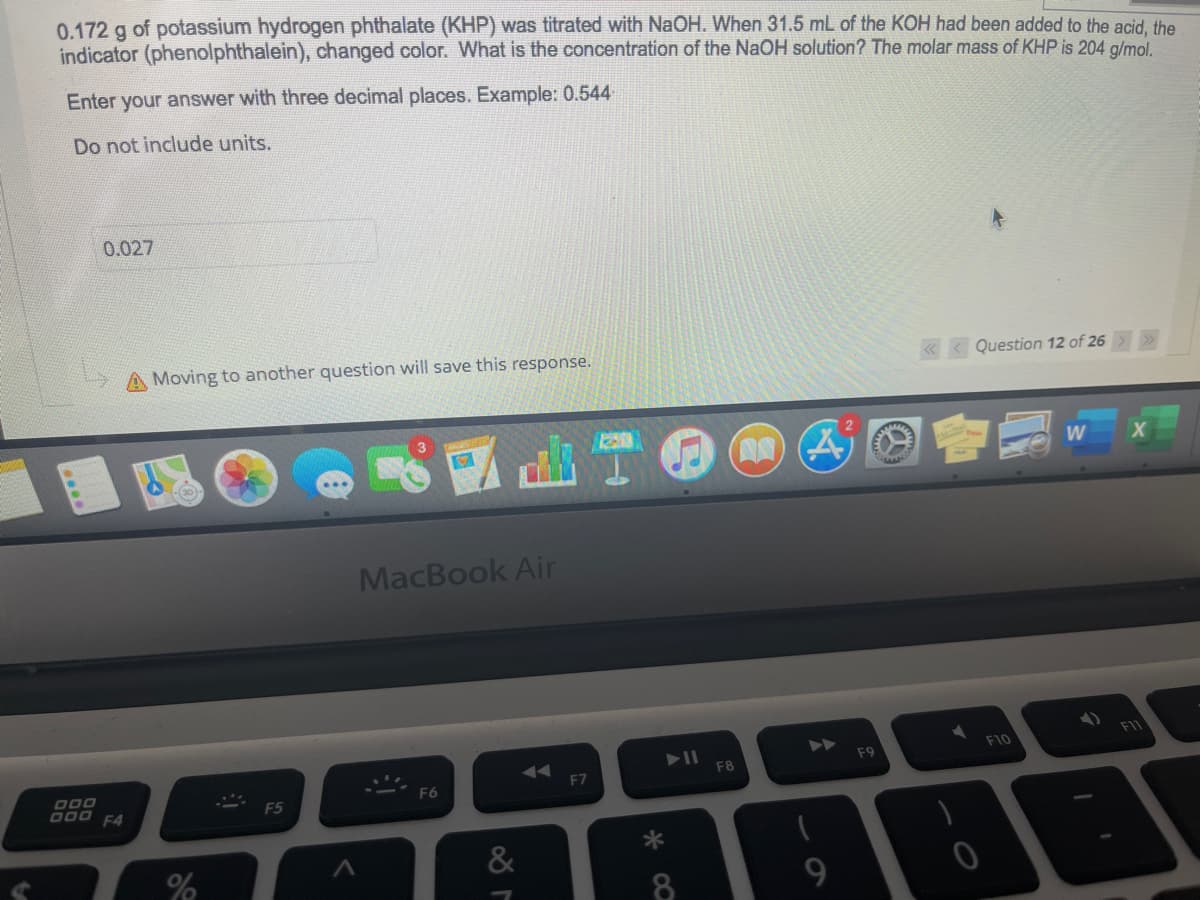
Transcribed Image Text:0.172 g of potassium hydrogen phthalate (KHP) was titrated with NaOH. When 31.5 mL of the KOH had been added to the acid. the
indicator (phenolphthalein), changed color. What is the concentration of the NaOH solution? The molar mass of KHP is 204 g/mol.
Enter your answer with three decimal places. Example: 0.544
Do not include units.
0.027
A Moving to another question will save this response.
Question 12 of 26
MacBook Air
F10
F9
F8
000
F7
F6
000 F4
F5
*
&
8.
9.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
