To measure the amount of nickel in some industrial waste fluid, an analytical chemist adds 0.1700M sodium hydroxide (NaOH) solution to a 23.00 g sample of the fluid and collects the solid nickel(II) hydroxide (Ni (OH),) product. When no more Ni(OH), is produced, she filters, washes and weighs it, and finds that 0.30 g has been produced. The balanced chemical equation for the reaction is: Ni?* (aq) + 2NaOH(aq) → Ni (OH), (s) + 2 Na* (aq) precipitation What kind of reaction is this? acid-base O redox If you said this was a precipitation reaction, enter the chemical formula of the precipitate. If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of Ni in the sample. Be sure your answer has the correct number of significant digits.
To measure the amount of nickel in some industrial waste fluid, an analytical chemist adds 0.1700M sodium hydroxide (NaOH) solution to a 23.00 g sample of the fluid and collects the solid nickel(II) hydroxide (Ni (OH),) product. When no more Ni(OH), is produced, she filters, washes and weighs it, and finds that 0.30 g has been produced. The balanced chemical equation for the reaction is: Ni?* (aq) + 2NaOH(aq) → Ni (OH), (s) + 2 Na* (aq) precipitation What kind of reaction is this? acid-base O redox If you said this was a precipitation reaction, enter the chemical formula of the precipitate. If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of Ni in the sample. Be sure your answer has the correct number of significant digits.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.88PAE: 4.88 A quality control technician needs to determine the percentage of arsenic (As) in a particular...
Related questions
Question
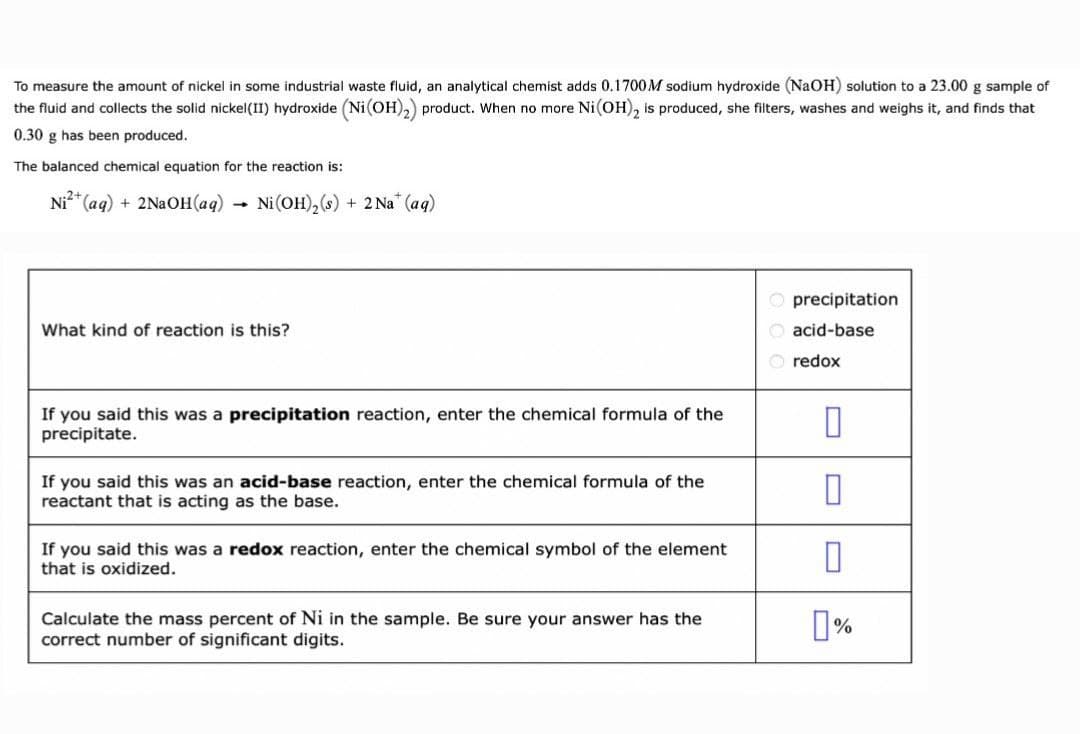
Transcribed Image Text:To measure the amount of nickel in some industrial waste fluid, an analytical chemist adds 0.1700M sodium hydroxide (NaOH) solution to a 23.00 g sample of
the fluid and collects the solid nickel(II) hydroxide (Ni(OH),) product. When no more Ni(OH), is produced, she filters, washes and weighs it, and finds that
0.30 g has been produced.
The balanced chemical equation for the reaction is:
Ni* (aq) + 2N2OH(aq) → Ni(OH),(s) + 2 Na" (aq)
O precipitation
What kind of reaction is this?
O acid-base
redox
If you said this was a precipitation reaction, enter the chemical formula of the
precipitate.
If you said this was an acid-base reaction, enter the chemical formula of the
reactant that is acting as the base.
If you said this was a redox reaction, enter the chemical symbol of the element
that is oxidized.
Calculate the mass percent of Ni in the sample. Be sure your answer has the
correct number of significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning