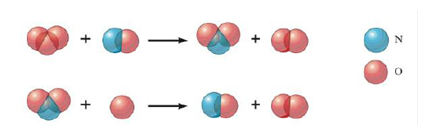
A proposed two-step mechanism for the destruction of ozone in the upper atmosphere is
a. What is the overall balanced equation for the ozone destruction reaction?
b. Which species is a catalyst?
c. Which species is an intermediate?
d. What is the rate law derived from this mechanism if the first step in the mechanism is slow and the second step is fast?
e. One of the concerns about the use of Freons is that they will migrate to the upper atmosphere, where chlorine atoms can be generated by the reaction
Chlorine atoms also can act as a catalyst for the destruction of ozone. The first step of a proposed mechanism for chlorine-catalyzed ozone destruction is
Cl (g) + O3 (g) → ClO (g) + O2 (g) Slow
Assuming a two-step mechanism, propose the second step in the mechanism and give the overall balanced equation.
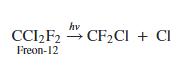
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 8 images









