Н-Н H-CI 436 kJ/mol С-Н 413 kJ/mol C=C 614 kJ/mol 431 kJ/mol C-C 348 kJ/mol 839 kJ/mol 567 kJ/mol Н-F N-H C-N С-О 293 kJ/mol 358 kJ/mol 799 kJ/mol 495 kJ/mol 391 kJ/mol N-O O-H O-0 F-F 201 kJ/mol С-F 485 kJ/mol 1072 kJ/mol 463 kJ/mol C-CI 328 kJ/mol C=N 615 kJ/mol 146 kJ/mol C-S 259 kJ/mol N=N 418 kJ/mol 155 kJ/mol Cl-CI 242 kJ/mol N=N 941 kJ/mol C=N 891 kJ/mol Estimate the enthalpy change (A Hrxn) of the following reaction for 2 moles of Cl2 using the bond energies above. H-H + CI-CI → H-CI + H-CI kJ
Н-Н H-CI 436 kJ/mol С-Н 413 kJ/mol C=C 614 kJ/mol 431 kJ/mol C-C 348 kJ/mol 839 kJ/mol 567 kJ/mol Н-F N-H C-N С-О 293 kJ/mol 358 kJ/mol 799 kJ/mol 495 kJ/mol 391 kJ/mol N-O O-H O-0 F-F 201 kJ/mol С-F 485 kJ/mol 1072 kJ/mol 463 kJ/mol C-CI 328 kJ/mol C=N 615 kJ/mol 146 kJ/mol C-S 259 kJ/mol N=N 418 kJ/mol 155 kJ/mol Cl-CI 242 kJ/mol N=N 941 kJ/mol C=N 891 kJ/mol Estimate the enthalpy change (A Hrxn) of the following reaction for 2 moles of Cl2 using the bond energies above. H-H + CI-CI → H-CI + H-CI kJ
Chapter13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy
Section13.SE: Something Extra
Problem 33AP
Related questions
Question
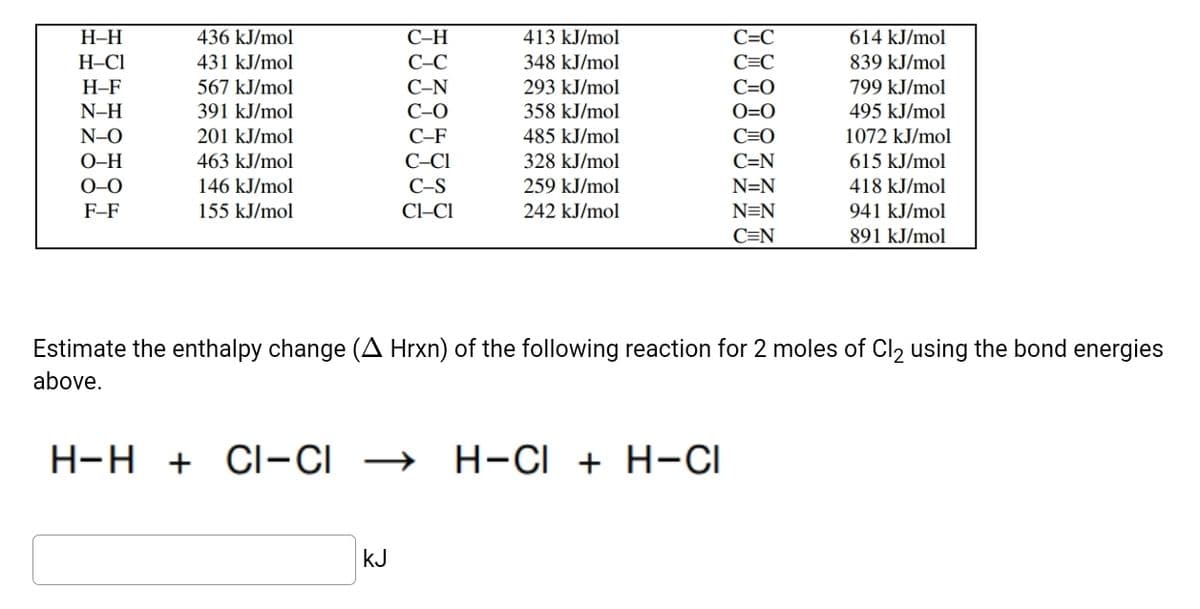
Transcribed Image Text:Н-Н
436 kJ/mol
С-Н
413 kJ/mol
C=C
614 kJ/mol
H-CI
431 kJ/mol
C-C
348 kJ/mol
C=C
839 kJ/mol
293 kJ/mol
C=0
O=0
Н-F
567 kJ/mol
С-N
799 kJ/mol
N-H
391 kJ/mol
C-O
358 kJ/mol
495 kJ/mol
N-O
201 kJ/mol
С-F
485 kJ/mol
C=0
1072 kJ/mol
О-Н
463 kJ/mol
C-CI
328 kJ/mol
C=N
615 kJ/mol
0–0
146 kJ/mol
C-S
259 kJ/mol
N=N
418 kJ/mol
F-F
155 kJ/mol
Cl-CI
242 kJ/mol
N=N
941 kJ/mol
C=N
891 kJ/mol
Estimate the enthalpy change (A Hrxn) of the following reaction for 2 moles of Cl2 using the bond energies
above.
H-H
+ Cl-CI
H-CI + H-CI
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

