Na Mg 38 48 58 68 78 88 – 18 28 AI SI P S CI Ar Arrange the following ions in order of decreasing ionization energy: Sr2+, Se2-, Rb+, Br 1A 7A 8A H 2A IMetals H He Each element has a name and a 3A 4A 5A 6A IMetalloids symbol. Notice that only the first letter of an element's symbol is capitalized - cobalt is Co, not CO. Chemists have arranged the elements in a table that depicts the symbols for the elements along with other information. This is called the periodic table. LI Be INonmetals BCNO F Ne Na Mg 38 4B 58 6B 7B 88 - 18 28 Al Si PS CI Ar K Ca Sc TI V Cr Mn Fe Co NI Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb BI Po At Rn Fr Ra Ac Rf Db Lanthanides Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Actinides Th Pa U Np Pu Am Cm Bk cf Es Fm Md No Lr Decreasing ionization energy Drag and drop your selection from the following list to complete the answer: Br Sr2+ Se2- Rb+ Submit Answer Try Another Version 1 item attempt remaining
Na Mg 38 48 58 68 78 88 – 18 28 AI SI P S CI Ar Arrange the following ions in order of decreasing ionization energy: Sr2+, Se2-, Rb+, Br 1A 7A 8A H 2A IMetals H He Each element has a name and a 3A 4A 5A 6A IMetalloids symbol. Notice that only the first letter of an element's symbol is capitalized - cobalt is Co, not CO. Chemists have arranged the elements in a table that depicts the symbols for the elements along with other information. This is called the periodic table. LI Be INonmetals BCNO F Ne Na Mg 38 4B 58 6B 7B 88 - 18 28 Al Si PS CI Ar K Ca Sc TI V Cr Mn Fe Co NI Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb BI Po At Rn Fr Ra Ac Rf Db Lanthanides Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Actinides Th Pa U Np Pu Am Cm Bk cf Es Fm Md No Lr Decreasing ionization energy Drag and drop your selection from the following list to complete the answer: Br Sr2+ Se2- Rb+ Submit Answer Try Another Version 1 item attempt remaining
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter8: Electron Configurations And Periodicity
Section: Chapter Questions
Problem 8.27QP
Related questions
Question
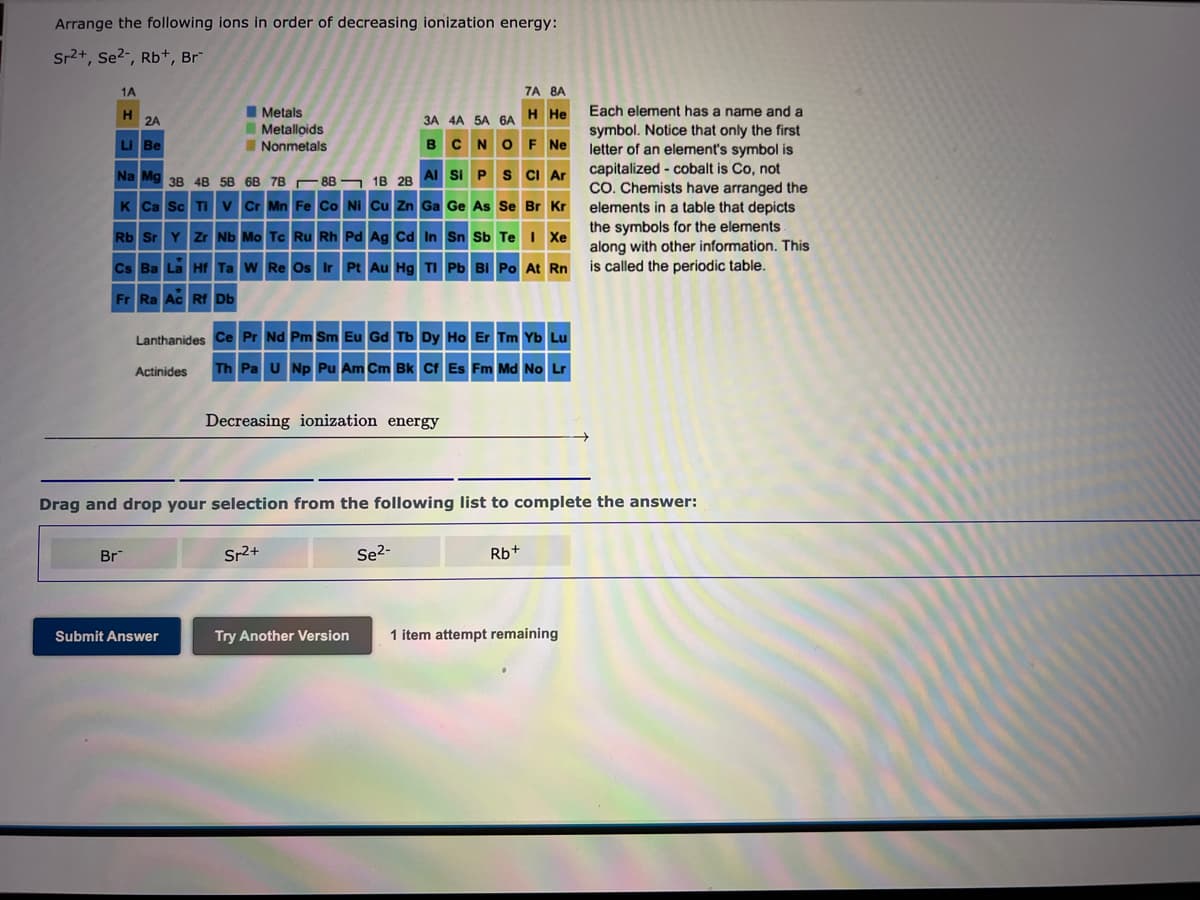
Transcribed Image Text:Arrange the following ions in order of decreasing ionization energy:
Sr2+, Se2-, Rb+, Br
1A
7A 8A
H
2A
I Metals
Each element has a name and a
ЗА 4А 5A 6А Н Не
IMetalloids
symbol. Notice that only the first
letter of an element's symbol is
capitalized - cobalt is Co, not
CO. Chemists have arranged the
elements in a table that depicts
the symbols for the elements
along with other information. This
is called the periodic table.
LI Be
INonmetals
BCNOF Ne
Na Mg 38 4B 5B 6B 7B 8B ¬ 1B 2B Al Si P S CI Ar
K Ca Sc TI v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn
Fr Ra Ac RE Db
Lanthanides Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Actinides
Th Pa U Np Pu Am Cm Bk cf Es Fm Md No Lr
Decreasing ionization energy
Drag and drop your selection from the following list to complete the answer:
Br
Sr2+
Se2-
Rb+
Submit Answer
Try Another Version
1 item attempt remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning