Name Element "Teams' Similar to teams of ballplayers, elements have symbols (abbreviations or logos) and are arranged in groups (like leagues) and periods (like divisions). And like ballplayers, when discussing elements, it's important to recognize their symbol, group and period. A. For each of the following elements, check the Periodic Table and write the element's symbol, group, and period. The first is done for you. Element Name Element Symbol Period Group Number Number Element Element Group Period Name Symbol Number Number chlorine CI krypton calcium potassium radium iodine xenon strontium beryllium tin cesium sulfur oxygen boron hydrogen carbon lithium aluminum bromine rubidium radon lead sodium arsenic bismuth nitrogen Grp 1 Per 3 Grp 8 Per 3 Grp 8 Per 2 Grp 5 Grp 6 Grp 7 Per 2 Grp 3 Per 2 Per 7 Per 6 DAL!
Name Element "Teams' Similar to teams of ballplayers, elements have symbols (abbreviations or logos) and are arranged in groups (like leagues) and periods (like divisions). And like ballplayers, when discussing elements, it's important to recognize their symbol, group and period. A. For each of the following elements, check the Periodic Table and write the element's symbol, group, and period. The first is done for you. Element Name Element Symbol Period Group Number Number Element Element Group Period Name Symbol Number Number chlorine CI krypton calcium potassium radium iodine xenon strontium beryllium tin cesium sulfur oxygen boron hydrogen carbon lithium aluminum bromine rubidium radon lead sodium arsenic bismuth nitrogen Grp 1 Per 3 Grp 8 Per 3 Grp 8 Per 2 Grp 5 Grp 6 Grp 7 Per 2 Grp 3 Per 2 Per 7 Per 6 DAL!
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 103GQ
Related questions
Question
( do both plzz both are connected )
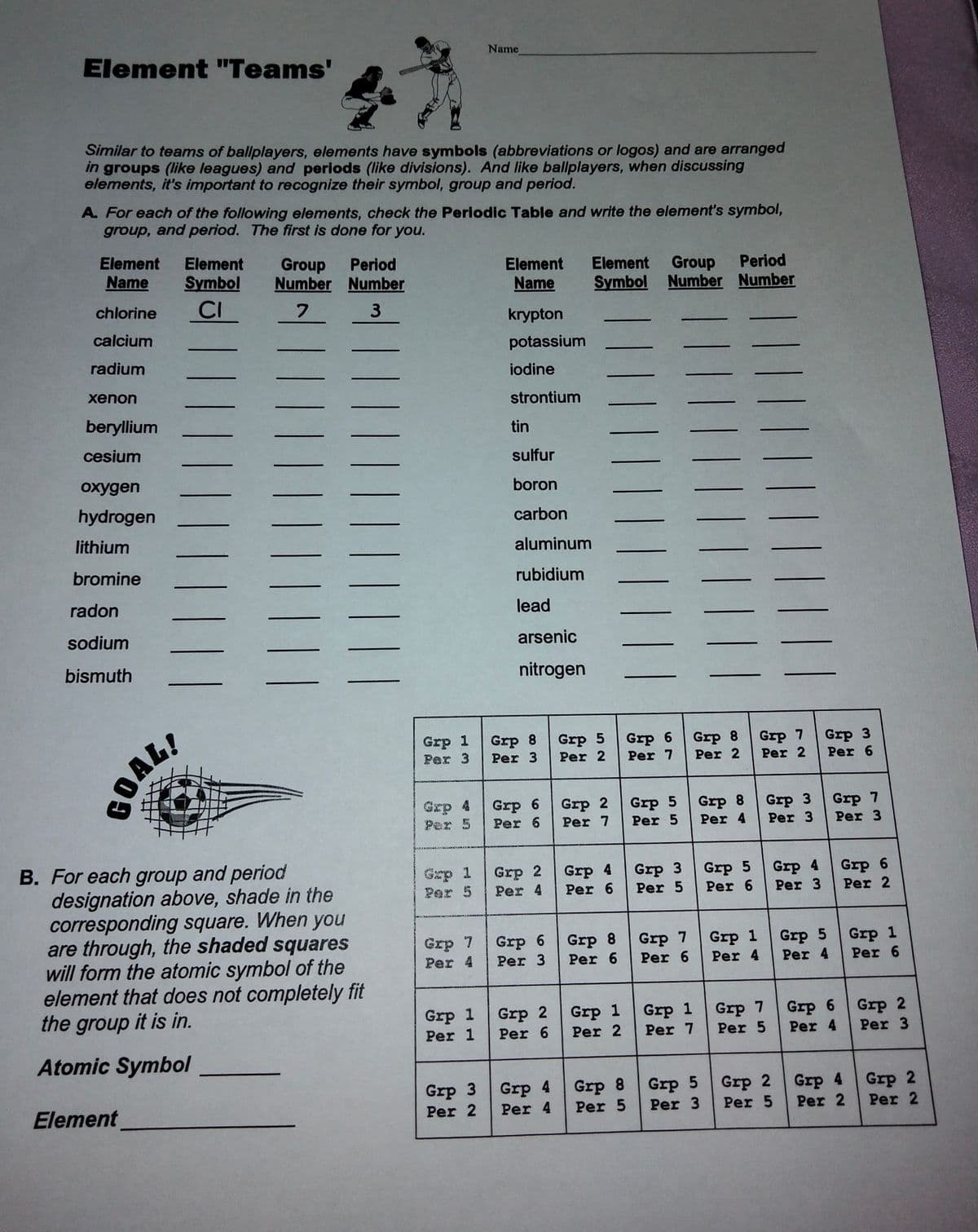
Transcribed Image Text:Element "Teams'
Name
Similar to teams of ballplayers, elements have symbols (abbreviations or logos) and are arranged
in groups (like leagues) and periods (like divisions). And like ballplayers, when discussing
elements, it's important to recognize their symbol, group and period.
A. For each of the following elements, check the Periodic Table and write the element's symbol,
group, and period. The first is done for you.
Element
Element
Period
Group
Number Number
Name
Element
Element Group
Period
Number Number
Symbol
Name Symbol
chlorine
CI
7.
krypton
calcium
potassium
radium
iodine
xenon
strontium
beryllium
tin
cesium
sulfur
oxygen
boron
hydrogen
carbon
lithium
aluminum
bromine
rubidium
radon
lead
sodium
arsenic
bismuth
nitrogen
Grp 1
Per 3
Grp 8
Per 3
Grp 5
Per 2
Grp 6
Per 7
Grp 8
Grp 7
Per 2
Grp 3
Per 6
AL!
Per 2
Grp 4
Per 5
Grp 6
Per 6
Grp 2
Grp 5
Рer 5
Grp 3
Per 3
Grp 8
Grp 7
Per 3
Per 7
Per 4
B. For each group and period
designation above, shade in the
corresponding square. When you
are through, the shaded squares
will form the atomic symbol of the
element that does not completely fit
the group it is in.
Grp 1
Per 5
Grp 2
Per 4
Grp 4
Per 6
Grp 3
Per 5
Grp 5
Per 6
Grp 4
Per 3
Grp 6
Per 2
Grp 7
Per 4
Grp 6
Per 3
Grp 7
Per 6
Grp 8
Grp 5
Per 4
Grp 1
Grp 1
Per 6
Per 6
Per 4
Grp 1
Per 1
Grp 1
Per 2
Grp 2
Grp 1
Per 7
Grp 7
Per 5
Grp 6
Grp 2
Per 3
Per 6
Per 4
Atomic Symbol
Element
Grp 3
Per 2
Grp 4
Per 4
Grp 8
Per 5
Grp 5
Per 3
Grp 2
Per 5
Grp 4
Per 2
Grp 2
Per 2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
