Name: Section and Group: Date: Stoichiometry of Hydrate Decomposition - Lab Report Pay attention to the number of significant digits in the calculations. Observations Observations of fresh copper(II) sulfate hydrate Observations of heated copper(II) sulfate blue, griny/fng blee fine texture Light gn/gr tre locks lille ch grean ble/gray mted Celer / fine Rxfure poder Trial 1 Trial 2 Data Record 3 decimal places Mass of evaporating dish 51.9179 51.921 Mass fresh copper(II) sulfate hydrate 1,015g -717g Mass of dish and heated 52.561g 52.3709 copper(II) sulfate Calculations Record 3 decimal places Mass heated copper(Il) - 449.9 |59.629/mal 159.62g/md Moles of copper(I) sulfate 4coRlaiotacl_2.81 x10 mel for masses sulfate Molar mass of anhydrous (no water) copper(II) sulfate Record 3 sig figs for calculations (after heating) Mass of water lost (= mass of fresh copper(II) sulfate – mass of heated 3419 371 268 copper(II) sulfate) 18.0Ve glmol 18.016glmdl Molar mass of water -2 Moles of water lost -371 Your ratio: Your ratio: 18-016 Ratio of moles of water/ 5.1l mélll 5.30mel 5-al mel moles of copper(II) sulfate Average ratio:
Name: Section and Group: Date: Stoichiometry of Hydrate Decomposition - Lab Report Pay attention to the number of significant digits in the calculations. Observations Observations of fresh copper(II) sulfate hydrate Observations of heated copper(II) sulfate blue, griny/fng blee fine texture Light gn/gr tre locks lille ch grean ble/gray mted Celer / fine Rxfure poder Trial 1 Trial 2 Data Record 3 decimal places Mass of evaporating dish 51.9179 51.921 Mass fresh copper(II) sulfate hydrate 1,015g -717g Mass of dish and heated 52.561g 52.3709 copper(II) sulfate Calculations Record 3 decimal places Mass heated copper(Il) - 449.9 |59.629/mal 159.62g/md Moles of copper(I) sulfate 4coRlaiotacl_2.81 x10 mel for masses sulfate Molar mass of anhydrous (no water) copper(II) sulfate Record 3 sig figs for calculations (after heating) Mass of water lost (= mass of fresh copper(II) sulfate – mass of heated 3419 371 268 copper(II) sulfate) 18.0Ve glmol 18.016glmdl Molar mass of water -2 Moles of water lost -371 Your ratio: Your ratio: 18-016 Ratio of moles of water/ 5.1l mélll 5.30mel 5-al mel moles of copper(II) sulfate Average ratio:
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question
Can you check to make sure my calculations are according to the data I obtained. Thanks
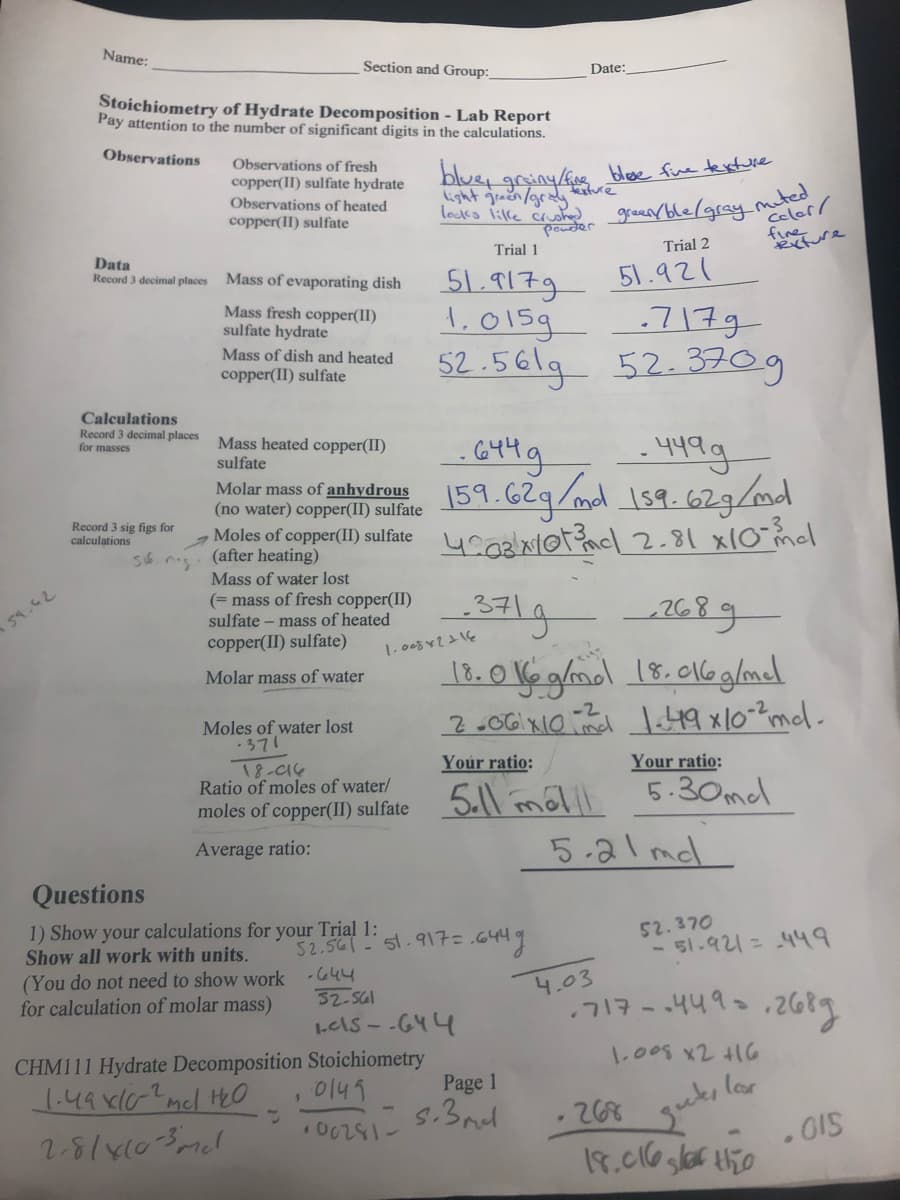
Transcribed Image Text:Name:
Section and Group:
Date:
Stoichiometry of Hydrate Decomposition - Lab Report
Pay attention to the number of significant digits in the calculations.
Observations
blue, griny/fne blese fue texture
sture
Observations of fresh
copper(II) sulfate hydrate
geanrble/gray.mted
celer /
ight graen/grady
Observations of heated
copper(II) sulfate
locks lilke ched
pouder
Trial 1
Trial 2
Data
Record 3 decimal places
51.917g
51.921
Mass of evaporating dish
Mass fresh copper(II)
sulfate hydrate
1,015g
-717g
Mass of dish and heated
52.561g 52.3709
copper(II) sulfate
Calculations
Record 3 decimal places
for masses
- 449g
Molar mas of anhudrus 59.629/ml 159. 62g/md
Mass heated copper(II)
sulfate
Molar mass of anhydrous
(no water) copper(II) sulfate
Record 3 sig figs for
calculations
Moles of copper(II) sulfate
(after heating)
Mass of water lost
(= mass of fresh copper(II)
sulfate – mass of heated
59.42
copper(II) sulfate)
18.0 Ve ghol 18.016 glmdl
Molar mass of water
-2
nd
Moles of water lost
371
T8-a4
Ratio of moles of water/
moles of copper(II) sulfate
Your ratio:
Your ratio:
5.30ml
5.11 métll
5-21 mdl
Average ratio:
Questions
1) Show your calculations for your Trial 1:
Show all work with units.
52.370
- 51.921=449
52.5af :' st.91구= .G44 9
(You do not need to show work -444
for calculation of molar mass)
4.03
.717-.449
32-SGl
LelS - -G44
1.008 x2 416
CHM111 Hydrate Decomposition Stoichiometry
Page 1
이141
1.49 xl02mcl HO
2-8/x103mcl
5.3nl
268
. O15
18.016 glar thio
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you



