The mixture that you will separate contains three components: NaCl, NH4CI, and SiO2. Their separation will be accomplished by heating the mixture to sublime the NH4CI, extracting the NaCl with water, and finally drying the remaining SiO2, as illustrated in the scheme shown in Figure 1. Figure 1: Flow diagram for the separation of the components of a mixture. Mixture: NH4CI NaCl NH CI sublimes Measure NH CI content by mass loss Heat to 520°C SiO2 Residue remaining after heating NaCl SiO2 Extract with H20 Residue from extraction NaCl Wet solution SiO2 Heat to dryness Heat to dryness bar NaCl SiO2 So Weigh NaCl Weigh SiO2 Laboratory Questions 1. Based on the procedure of this experiment, explain the effect of the following actions on the the percent recovery. The choices include: lower, higher, no effect or could be higher or measured masses of the components indicated. Where indicated, also address the effect on lower. If the answer could be higher or lower, provide a detailed explanation. a) Sublimation of NH4CI was not complete. How does this situation affect the measured mass of NH4CI and the percent recovery? b) While decanting, some of the salty water was spilled onto the lab bench. How does this situation affect the measured mass of NaCl and the percent recovery? c) While decanting, some of the sand was inadvertently transferred to the second evaporating dish. How does this situation affect the measured masses of NaCl and SiO2 and the percent recovery? d) While weighing the first evaporating dish after subliming the NH4CI, the balance was not properly set to zero prior to the measurement. How does this situation affect the measured mass of NH4CI? e While drying the NaCl, some of the solution splashed onto the watch glass causing salt to deposit on the watch glass. How does this situation affect the measured mass of NaCl? f) While drying the sand, formation of steam caused some the sand to eject out of the container and onto the lab bench. How does this situation affect the measured mass of SiO2 and the percent recovery? 2. Could the separation in this experiment have been done ina different order? For example, if the mixture was first extracted with water and then the extract and the insoluble residue both heated to dryness, could you determine the amounts of NaCl, NH4CI, and SiO2 originally present? Note that both NaCl and NH4CI are soluble in water. Based on the interpretation, there could be more than one correct answer. Your answer should be justified with a detailed explanation.
The mixture that you will separate contains three components: NaCl, NH4CI, and SiO2. Their separation will be accomplished by heating the mixture to sublime the NH4CI, extracting the NaCl with water, and finally drying the remaining SiO2, as illustrated in the scheme shown in Figure 1. Figure 1: Flow diagram for the separation of the components of a mixture. Mixture: NH4CI NaCl NH CI sublimes Measure NH CI content by mass loss Heat to 520°C SiO2 Residue remaining after heating NaCl SiO2 Extract with H20 Residue from extraction NaCl Wet solution SiO2 Heat to dryness Heat to dryness bar NaCl SiO2 So Weigh NaCl Weigh SiO2 Laboratory Questions 1. Based on the procedure of this experiment, explain the effect of the following actions on the the percent recovery. The choices include: lower, higher, no effect or could be higher or measured masses of the components indicated. Where indicated, also address the effect on lower. If the answer could be higher or lower, provide a detailed explanation. a) Sublimation of NH4CI was not complete. How does this situation affect the measured mass of NH4CI and the percent recovery? b) While decanting, some of the salty water was spilled onto the lab bench. How does this situation affect the measured mass of NaCl and the percent recovery? c) While decanting, some of the sand was inadvertently transferred to the second evaporating dish. How does this situation affect the measured masses of NaCl and SiO2 and the percent recovery? d) While weighing the first evaporating dish after subliming the NH4CI, the balance was not properly set to zero prior to the measurement. How does this situation affect the measured mass of NH4CI? e While drying the NaCl, some of the solution splashed onto the watch glass causing salt to deposit on the watch glass. How does this situation affect the measured mass of NaCl? f) While drying the sand, formation of steam caused some the sand to eject out of the container and onto the lab bench. How does this situation affect the measured mass of SiO2 and the percent recovery? 2. Could the separation in this experiment have been done ina different order? For example, if the mixture was first extracted with water and then the extract and the insoluble residue both heated to dryness, could you determine the amounts of NaCl, NH4CI, and SiO2 originally present? Note that both NaCl and NH4CI are soluble in water. Based on the interpretation, there could be more than one correct answer. Your answer should be justified with a detailed explanation.
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
Can someone answer number 2 please
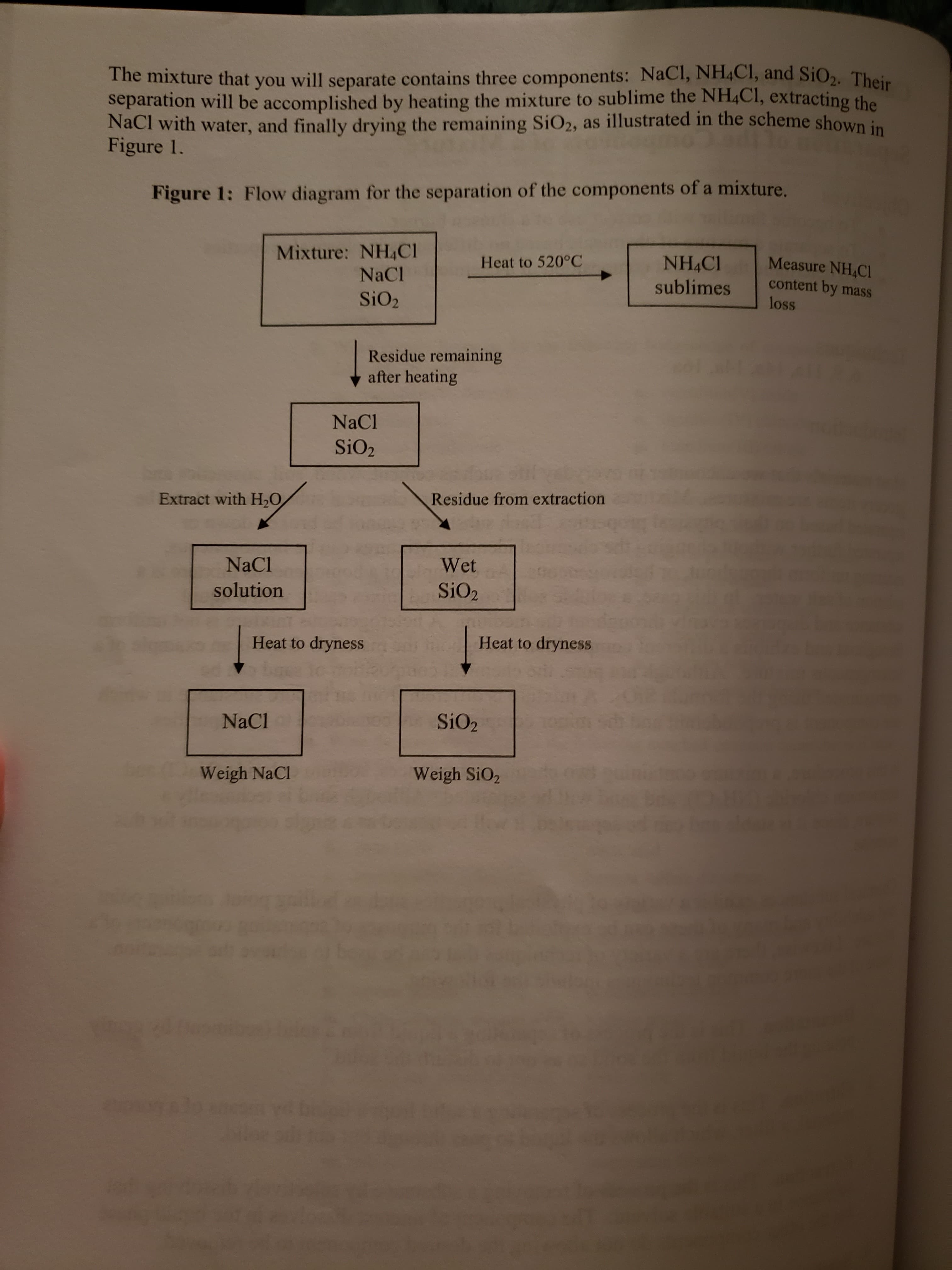
Transcribed Image Text:The mixture that you will separate contains three components: NaCl, NH4CI, and SiO2. Their
separation will be accomplished by heating the mixture to sublime the NH4CI, extracting the
NaCl with water, and finally drying the remaining SiO2, as illustrated in the scheme shown in
Figure 1.
Figure 1: Flow diagram for the separation of the components of a mixture.
Mixture: NH4CI
NaCl
NH CI
sublimes
Measure NH CI
content by mass
loss
Heat to 520°C
SiO2
Residue remaining
after heating
NaCl
SiO2
Extract with H20
Residue from extraction
NaCl
Wet
solution
SiO2
Heat to dryness
Heat to dryness
bar
NaCl
SiO2
So
Weigh NaCl
Weigh SiO2

Transcribed Image Text:Laboratory Questions
1. Based on the procedure of this experiment, explain the effect of the following actions on the
the percent recovery. The choices include: lower, higher, no effect or could be higher or
measured masses of the components indicated. Where indicated, also address the effect on
lower. If the answer could be higher
or lower, provide a detailed explanation.
a) Sublimation of NH4CI was not complete. How does this situation affect the measured
mass of NH4CI and the percent recovery?
b) While decanting, some of the salty water was spilled onto the lab bench. How does this
situation affect the measured mass of NaCl and the percent recovery?
c) While decanting, some of the sand was inadvertently transferred to the second
evaporating dish. How does this situation affect the measured masses of NaCl and SiO2
and the percent recovery?
d) While weighing the first evaporating dish after subliming the NH4CI, the balance was not
properly set to zero prior to the measurement. How does this situation affect the
measured mass of NH4CI?
e While drying the NaCl, some of the solution splashed onto the watch glass causing salt to
deposit on the watch glass. How does this situation affect the measured mass of NaCl?
f) While drying the sand, formation of steam caused some the sand to eject out of the
container and onto the lab bench. How does this situation affect the measured mass of
SiO2 and the percent recovery?
2. Could the separation in this experiment have been done ina different order? For example, if
the mixture was first extracted with water and then the extract and the insoluble residue both
heated to dryness, could you determine the amounts of NaCl, NH4CI, and SiO2 originally
present? Note that both NaCl and NH4CI are soluble in water. Based on the interpretation,
there could be more than one correct answer. Your answer should be justified with a detailed
explanation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning