Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.8QAP
Related questions
Question
Need assistance with finding the density of water and all
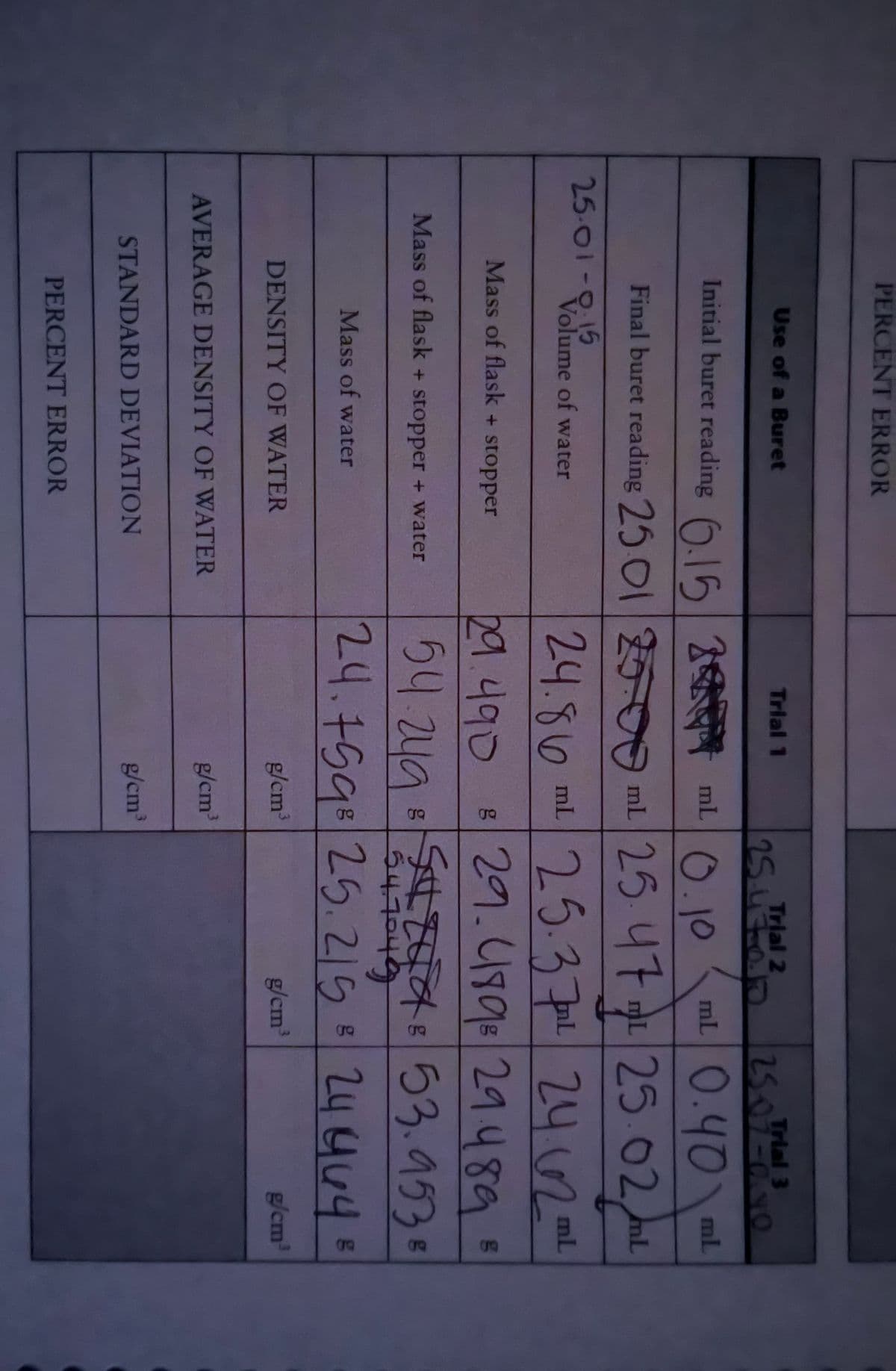
Transcribed Image Text:PERCENT ERROR
Trial 3
25.07-000
Use of a Buret
Trial 1
Trial 2
Initial buret reading 6.5
2 ml.
ml 0.40\
mL
0ml 25 47 L 25.02m
25.02/mL
Final buret reading 25 01
25.01-0.15
24.86ml ul l
29.49929.489
25.37
2462=
Volume of water
Mass of flask + stopper
29.4908
54.2yg
24¢
53.9535
Mass of flask + stopper + water
24.759825.215 B
24464¢
Mass of water
DENSITY OF WATER
g/cm³
g/cm³
g/cm
AVERAGE DENSITY OF WATER
g/cm
STANDARD DEVIATION
g/cm
PERCENT ERROR
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



