Chapter11: Dynamic Electrochemistry
Section: Chapter Questions
Problem 3P
Related questions
Question
Need help with the last question
Transcribed Image Text:Not Secure - a5.chem.binghamton.edu C
E LON-CAP.
MC Kimetsu...
M Mathway.
G 25 ml to I..
Chem 10...
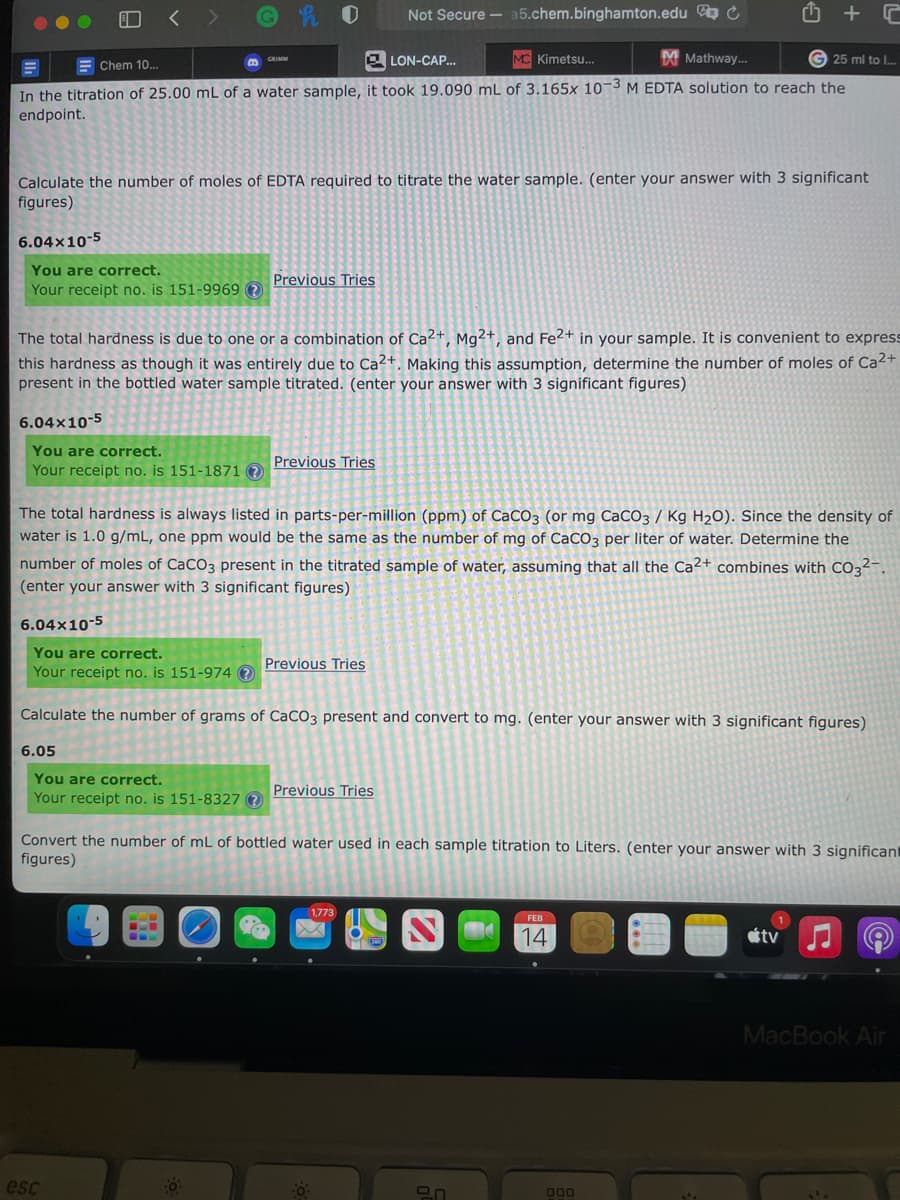
In the titration of 25.00 mL of a water sample, it took 19.090 mL of 3.165x 10-3 M EDTA solution to reach the
endpoint.
Calculate the number of moles of EDTA required to titrate the water sample. (enter your answer with 3 significant
figures)
6.04x10-5
You are correct.
Your receipt no. is 151-9969 2
Previous Tries
The total hardness is due to one or a combination of Ca2+, Mg²+, and Fe2+ in your sample. It is convenient to express
this hardness as though it was entirely due to Ca²+. Making this assumption, determine the number of moles of Ca2+
present in the bottled water sample titrated. (enter your answer with 3 significant figures)
6.04x10-5
You are correct.
Your receipt no. is 151-1871 ?
Previous
The total hardness is always listed in parts-per-million (ppm) of CaCO3 (or mg CaCO3 / Kg H2O). Since the density of
water is 1.0 g/mL, one ppm would be the same as the number of mg of CaCO3 per liter of water. Determine the
number of moles of CaCO3 present in the titrated sample of water, assuming that all the Ca2+ combines with CO3²-.
(enter your answer with 3 significant figures)
6.04x10-5
You are correct.
Previous Tries
Your receipt no. is 151-974 ?
Calculate the number of grams of CaCO3 present and convert to mg. (enter your answer with 3 significant figures)
6.05
You are correct.
Your receipt no. is 151-8327 ?
Previous Tries
Convert the number of mL of bottled water used in each sample titration to Liters. (enter your answer with 3 significant
figures)
1,773
FEB
14
étv
MacBook Air
esc
000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning