Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 86AP
Related questions
Question
Please solve question 10 relate to question 9
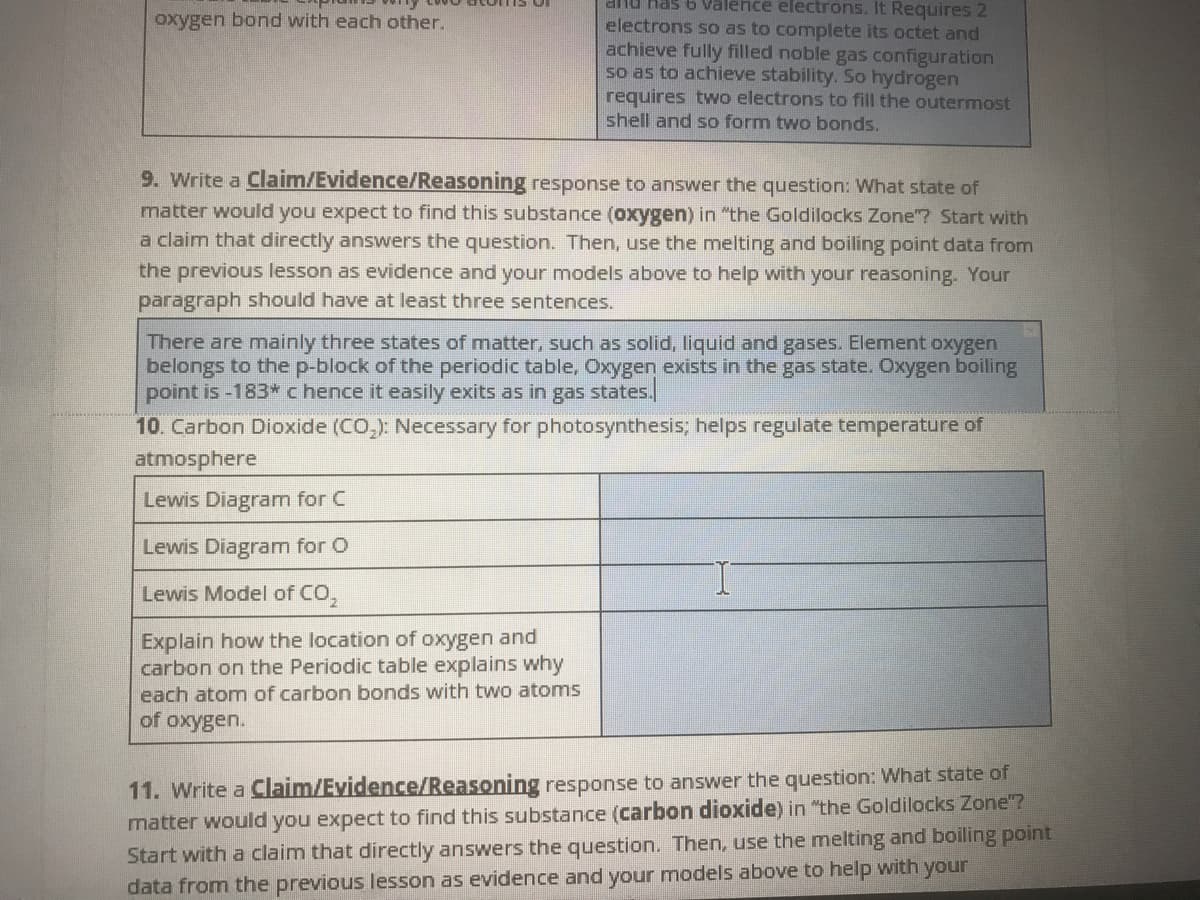
Transcribed Image Text:anu Has 6 Valence electrons. It Requires 2
oxygen bond with each other.
electrons so as to complete its octet and
achieve fully filled noble gas configuration
so as to achieve stability. So hydrogen
requires two electrons to fill the outermost
shell and so form two bonds.
9. Write a Claim/Evidence/Reasoning response to answer the question: What state of
matter would you expect to find this substance (0xygen) in "the Goldilocks Zone"? Start with
a claim that directly answers the question. Then, use the melting and boiling point data from
the previous lesson as evidence and your models above to help with your reasoning. Your
paragraph should have at least three sentences.
There are mainly three states of matter, such as solid, liquid and gases. Element oxygen
belongs to the p-block of the periodic table, Oxygen exists in the gas state. Oxygen boiling
point is -183* c hence it easily exits as in gas states.
10. Carbon Dioxide (CO,): Necessary for photosynthesis; helps regulate temperature of
atmosphere
Lewis Diagram for C
Lewis Diagram for O
Lewis Model of CO,
Explain how the location of oxygen and
carbon on the Periodic table explains why
each atom of carbon bonds with two atoms
of oxygen.
11. Write a Claim/Evidence/Reasoning response to answer the question: What state of
matter would you expect to find this substance (carbon dioxide) in "the Goldilocks Zone"?
Start with a claim that directly answers the question. Then, use the melting and boiling point
data from the previous lesson as evidence and your models above to help with your
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning
