Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.102PAE: 9.102 A runner generates 418 kJ of energy per kilometer from the cellular oxidation of food. The...
Related questions
Question
100%
need last one which is in red
Transcribed Image Text:PRINTER VERSeON
BACK
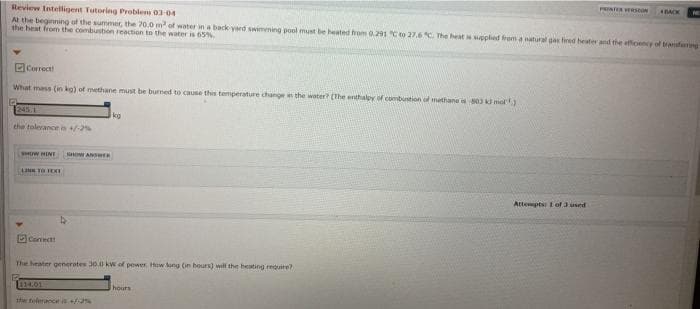
Review Intelligent Tutoring Problem 03-04
A the beginning of the summer, the 20.0 m of water in a back yard swimming pool must be heated hrom 0.291 Cto 27.6 The heat ppled from a natural gas fired heater and the efficency of tranferng
the heat from the combustion reaction to the water is 65%
DCorrect
What mass (in ka) of methane must be burmed to cause this temperature change i the water (The enthalpy of combustion of methana 80 mol)
1245.1
kg
the tolerance in -2%
INIH M
SHOW ANSWER
Attempts I of 3 used
Canect
The heater generotes 30.0 kW of power Hew tong (in hours) will the heating require?
1114.01
hours
the tolerance s+%
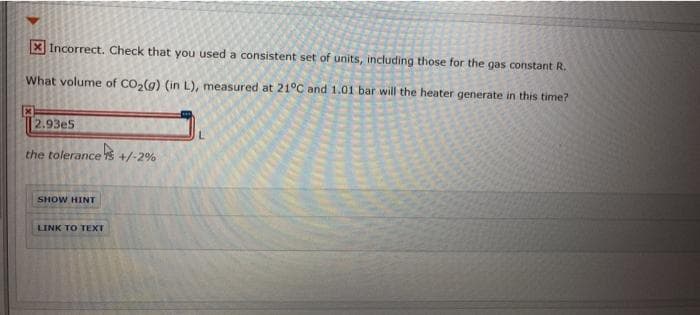
Transcribed Image Text:X Incorrect. Check that you used a consistent set of units, including those for the gas constant R.
What volume of CO,(g) (in L), measured at 21°C and 1.01 bar will the heater generate in this time?
2.93e5
the tolerance S +/-2%
SHOW HINT
LINK TO TEXT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,