Q: Consider the equilibrium system described by the chemical reaction below. A 1.00 L reaction vessel…
A:
Q: What pressure is required to achieve a CO2 concentration of 0.0690 mol L−1 at 20∘C?
A:
Q: . The dissociation of N;O4 into NO2 is 16.7% complete at 298 K and 1.013 bar. N2O«(g) = 2 NO2(g)…
A: Given : We have to calculate K and delta G
Q: For the reaction bellow, find the mole fractions of all spices. If KP=0.1 A 2B + C
A:
Q: 7) For a reaction Keg = 1.2 x 10-6 at T = 200 K. What is AG° for the reaction?
A:
Q: A chemist mixed 2.3 M of A, 1.6 M of B, and 0.7 Mof C to a sealed container at 750 K: A(g) + B(aq)…
A: Given reaction is : A (g) + B (aq) <------------->2C (g) , Kc = 9.8 x 10-1 Concentration of…
Q: 1. In a 10 L vessel at 1000 K, 20 g of solid carbon and CO, at 8.21 atm are introduced. Whan the…
A:
Q: The distribution of Na+ ions across a typical biological membrane is 10 mmol dm-3 inside the cell…
A: Given information: Distribution of Na+ ions inside cell of membrane is 10 mmol dm-3 and outside cell…
Q: why does the decomposition of calcium carbonate to calcium oxide and carbon dioxide occur in a…
A: The decomposition reaction is mostly activated by thermal energy. Decomposition of Calcium carbonate…
Q: Use the following data to estimate a value of Kp at 200 K for the reactionSingle choice.
A:
Q: DATA AND CALCULATIONS Volume (mL) Pressure (kPa) Constant, k (P/V or P V) 5,8. E.3 10.8 13.3 15.8…
A: As shown in question there are two possibililies of k, k= P/V k= P.V
Q: Substance A is 15 percent dissociated at 1732 K. and 1.00 bar, in the equilibrium: 2Ag Bg. Calculate…
A: The equilibrium reaction given is, => 2 A (g) → B (g) Given: At 1732 K and 1 bar, 15 % of A…
Q: In the gas-phase reaction A + B ⇋ C + 2 D, it was found that, when 2.00 mol A, 1.00 mol B, and 3.00…
A: A + B⇌ C + 2Dt=0 2mol 1mol 0 3molt=eqn 2-x 1-x 0+x…
Q: Chemistry What of the following options describes the term "dynamic equilibrium"? O a. A number…
A:
Q: Construct the expression for Kp for the following reaction. 2 CSH18(1) + 25 O2(g) = 16 CO2(g) + 18…
A: A reversible chemical reaction can move in either forward or backward direction. The stage of a…
Q: The decomposition of a generic diatomic element in its standard state is representdd by the equation…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: The equilbrium constant, Ke, for the following reaction is 0.215 at 673 K: NHẠI(s) NH3(g) + HI(g)…
A: Partial pressure of solid components is equal to one
Q: Calculate the Gibbs free Energy at 25°C from the Kf of Ag(NH3)2' using the equilibria 0.89 Ag+ (aq)…
A: Given : Ag+ (aq) + e- -->Ag(s) Ag(NH3)2+ + e- --> Ag(s) + 2NH3(aq)
Q: 2) Calculate the amount of limc(ca0) that Gon be propared by heating 200 kq of limestone that is 95…
A: CaCO3 ------------> CaO + CO2.
Q: The solubility of CaF, in water at 25°C is 2.05 x 10 mol. dm³. What is the of Ksp at 25°C?
A: Ksp is known as the solubility product constant. It is numerically equivalent to the product of the…
Q: The equilibrium constant for the gas phase reaction
A: Since we know that in a chemical reaction at equilibrium the rate of forward reaction become equal…
Q: The equiliorim constant Kc is 10.5 @350 K. 2 CH2 Clz (g)a CHy(G)+ CCl4 ) Calculate equililarium Con…
A: The chemical reaction taking place is: 2CH2Cl2g ⇔ CH4g + CCl4g The given information is: Kc = 10.5…
Q: The standard Gibbs energy of formation of gaseous ozone at 25.0 ℃ ΔfGo, is 162.3 kJ. mol-1, for…
A: The value of Kx at 2 bar
Q: Suppose 1.000 mol HgO(s) was placed in a tight, otherwise empty vessel, heated to 600 K, and kept…
A: Equilibrium constant in terms of pressure is defined as the product of partial pressures of products…
Q: The Ksp for a very insoluble salt is 4.2 * 10-47 at 298 K. What is ∆G° for the dissolution of the…
A: Ksp = 4.2 × 10-47 T = 298 K
Q: It was found that for the gas phase reaction: A +B + 2C + 3D when 15 mol A and 18 mol B were mixed…
A:
Q: how can the homogeneous equilibrium be attained by determining the equilibrium constant? explain in…
A: So, first we start from defining equilibrium state. The state of chemical reaction when thr…
Q: The decomposition of a generic diatomic element in its standard state is represented by the equation…
A: Solution- (a),At 2000K ∆G0f =5.51 kL/mol∆G0f = RT In KIn K=∆G0f-RTIn K = 5510J/mol-8.314J/mol K…
Q: Q2 // For the reaction bellow, find the mole fractions of all spices. If KP=0.3 A 2B + C
A: Given in following question a reaction A=2B+C calculate mole fraction of each species and also give…
Q: When adjusted for any changes in A,H and A,S with temperature, A;G° (500K) = 99.1kJ mol. Calculate…
A: The considered reaction is, CaCO3 (s)→CaO(s) + CO2 (g)
Q: Molecular bromine is 24 per cent dissociated at 1600 K and 1.00 bar in the equilibrium Br2(g) = 2…
A: Given: Degree of dissociation = 0.24 Initial pressure = 1 bar Temperature = 1600℃ = 1873.15K
Q: A mixture consisting of 1.000 mol H2O(g) and 1.000 mol CO(g) is placed ina reaction vessel of volume…
A: Using the relationship of Kp and Kc, value of Kp can be determined.
Q: e of Kp for t
A:
Q: .The equilibrium constant for the reaction of decomposition of AB3 (AB3(g) = A(g) + 3B (g)) is K =…
A: For a reaction , AB = A + B The dissociation constant of the reaction will be, k = [A][B] / [AB]…
Q: The solubility of ZnS(s) in water at a certain temperature is 1.4 × 10–11 mol/L, i.e., x or the…
A: Given solubility of ZnS(s) in water = 1.4 × 10-11 mol/L
Q: what is the value of Kp at 298 K?
A: Relationship between kp and kc :- kp = kc(RT)∆ng ∆ng is the change in gaseous moles in a reaction…
Q: A mixture consiting of 1.000 mol H2O(g) and 1.000 mol CO(g) is placed ina reaction vessel of volume…
A: Initial concentration of CO and H2O and also the concentration of CO2 at equilibrium can be…
Q: The equilibrium pressure of H2 on solid uranium and uranium hydride, UH3, is 139 Pa at 500 K.…
A:
Q: equilibrium constant K̟ for th
A:
Q: Consider a body of water in equilibrium with solid calcium sulfate, CaSO4, for which Ksp = 3.0 *…
A:
Q: For the following reaction, the degree of dissociation (a) of X2 is 39.0% at 1.2 bar total pressure…
A: At temperature T = 300 K Degree of dissociation (α) = 39.0 % Total pressure = 1.2 bar ∆Ho = 10.0…
Q: Use the data in the supporting materials to calculate the equilibrium constant (Kp) for the…
A: The solution is given in the following steps :
Q: Vapor pressure of water at 80c is 0.4675. Calculate in KPa
A: Answer :- 46.19 in KPa
Q: The decomposition of a generic diatomic element in its standard state is represented by the equation…
A: Given: At 2000 K, ΔG of the reaction = 5.55 KJ/mol = 5550 J/mol. And at 3000 K, ΔG of the reaction =…
Q: A mixture consiting of 1.000 mol H2O(g) and 1.000 mol CO(g) is placed ina reaction vessel of volume…
A: Given that,No.of moles of CO2 = 0.665 moleVolume of reaction vessel = 10 LThe reaction is
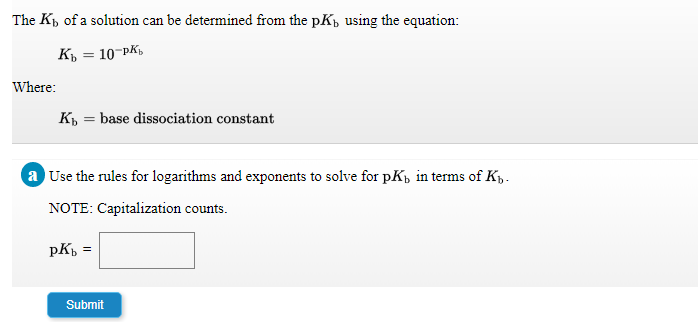
Step by step
Solved in 2 steps with 1 images

- Look up the appropriate Ka1 and Ka2 values for oxalic acid andascorbic acid, and repeat these calculations for these two weak, diprotic acids. Calculate the pH of both solutions and rank the acidities of the carbonic acid, oxalic acid, and ascorbic acidsolutions. In what ways is the ranking consistent with the magnitudes of the ionization constants?Oxalic acid (H2C2O4) is a diprotic acid with Ka1=5.6×10-1 and Ka2=1.5×10-4. Oxalate ion (C2O42-) also has two base dissociation constants, Kb(1) =6.5×10-11 and Kb2=1.8×10-14. Based on these values, deduce the formula to compute the base dissociation constants of oxalate ion in terms of Kw and Ka1 or Ka2.The base-dissociation constant, Kb, for pyridine, C5H5N is 1.4x10-9. The acid- dissociation constant, Ka for the pyridinium ion, C5H5NH+ is?
- In calculating pH by ICE method of 0.015 M CH2FCOOH (Ka CH2FCOOH = 2.6 x10-3), the change in CH2FCOOH can not be neglected because. a. Acid Dissociation constant, Ka, has a negative exponent. b. pH is not within +/- pka unit of pka of CH2FCOOH c. initial CH2FCOOH concentration is less than 0.1 M d. dissociation of [CH2FCOOH] will be more than 5% of initial [CH2FCOOH] ----------- To protect corrosion of materials made of iron, the surface of the iron material is electroplated with another metal. Given below information, which metal will be best to “galvanize” the material ? O2 + H2O + 2e- -> 4OH-(aq) .... E0 = 0.401 v Fe2+(g) + 2e- - -> Fe ............ E0 =(-) 0.440 v Cu2+(g) + 2e- - -> Cu ...........E0 = 0.337 v Ni2+(g) + 2e- - -> Ni ............ E0 = (-)0.250 v Zn2+(g) + 2e- - -> Zn ............ E0 =(-) 0.763 v a. Zn b. Ni c. Cu d. Fe2+ ------------ Given : C (s) + H2O(g) <-> CO(g) + H2(g) Which of the following will shift equilibria to form…Given that acetic acid has Ka = 1.8 x 10–5, what is the pH of a solution that contains the molar ratio of conjugate base-to-acid: [CH3CO2–]/[CH3CO2H] = 10/1?What are the fraction compositions of acid and conjugate base in a 0.0475 molar solution of propanoic acid?
- Given that the acid dissociation constant for benzoic acid (HOBz) is Ka= 6.5 x 10^−5. Calculate the basic dissociation constant, Kb of the benzoate ion (OBz−)The acid dissociation constant Ka of alloxanic acid HC4H3N2O5 is ×2.2410−7. Calculate the pH of a 1.2M solution of alloxanic acid. Round your answer to 1 decimal place.A solution is prepared by combining 20.0 mLs of 0.10 M solution of an unknown acid and 15.0 mLs of 0.20 M conjugate base. The measured pH of this solution is 8.43. Use this information to calculate the pKa of the acid
- Please don't provide handwritten solution... In the laboratory, a general chemistry student measured the pH of a 0.513 M aqueous solution of triethanolamine, C6H15O3N to be 10.721. Use the information she obtained to determine the Kb for this base.The acid dissociation constant Ka of hydrocyanic acid HCN is ×6.210−10. Calculate the pH of a 1.6M solution of hydrocyanic acid. Round your answer to 1 decimal place.Aqueous acids may be placed in order of acid strength according to their pka values. In the following, the stronger acids are nearer the top. HClO4(aq) ➡ H+(aq) + ClO-4(aq) HCl(aq) ➡ H+(aq) + Cl-aq) CH3CO2H(aq) ➡ CH3CO2-(aq) + H+(aq) H2O(l) ➡ H+(aq) + OH-(aq). Give one piece of experimental evidence to justify the above order. Define ka and pka

