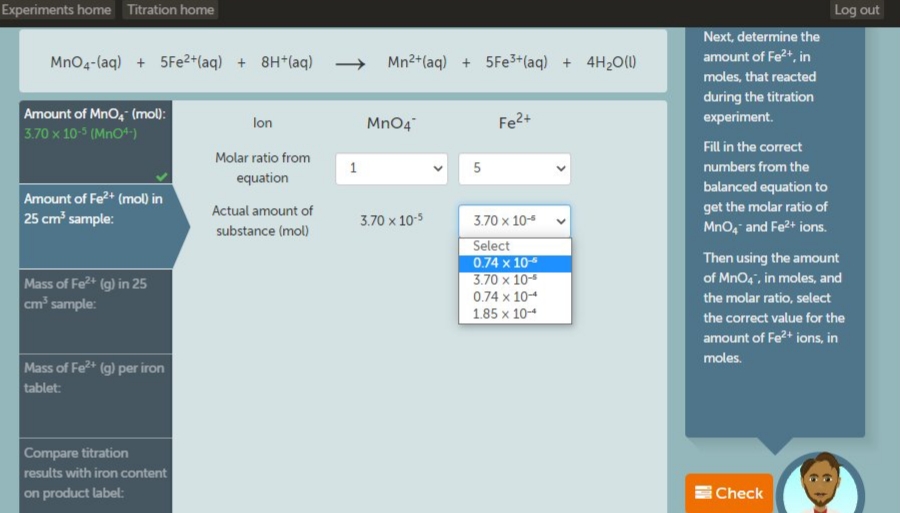
Next, determine th MnO4-(aq) + 5Fe2+(aq) + 8H*(aq) → Mn2+(aq) + 5Fe+(aq) + 4H20(1) amount of Fe2+, in moles, that reacted Amount of MnO, (mo0: 3.70 x 10- (Mno4) during the titration experiment. MnO4 Fe2+ lon Fill in the correct Molar ratio from numbers from the equation balanced equation Amount of Fe* (mol) in 25 cm' sample: Actual amount of get the molar ratio 3.70 x 103 3.70 x 104 substance (mol) Mno, and Fe2+ ion Select 0.74 x 10 3.70 x 10 0.74 x 104 1.85 x 104 Then using the ame of MnO,", in moles Mass of Fe (g) in 25 cm sample: the molar ratio, sel the correct value fo
Next, determine th MnO4-(aq) + 5Fe2+(aq) + 8H*(aq) → Mn2+(aq) + 5Fe+(aq) + 4H20(1) amount of Fe2+, in moles, that reacted Amount of MnO, (mo0: 3.70 x 10- (Mno4) during the titration experiment. MnO4 Fe2+ lon Fill in the correct Molar ratio from numbers from the equation balanced equation Amount of Fe* (mol) in 25 cm' sample: Actual amount of get the molar ratio 3.70 x 103 3.70 x 104 substance (mol) Mno, and Fe2+ ion Select 0.74 x 10 3.70 x 10 0.74 x 104 1.85 x 104 Then using the ame of MnO,", in moles Mass of Fe (g) in 25 cm sample: the molar ratio, sel the correct value fo
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.20QAP
Related questions
Question
select the correct answer
Transcribed Image Text:Experiments home Titration home
Log out
Next, determine the
amount of Fe2+, in
MnO4-(aq) + 5Fe2+(aq) + 8H*(aq) → Mn2+(aq) + 5FE3+(aq) + 4H20(1)
moles, that reacted
Amount of Mno, (mol):
3.70 x 10-5 (Mno+)
during the titration
experiment.
lon
Mn04
Fe2+
Fill in the correct
Molar ratio from
numbers from the
equation
balanced equation to
Amount of Fe2+ (mol) in
25 cm' sample:
Actual amount of
substance (mol)
get the molar ratio of
MnO, and Fe2+ ions.
3.70 x 10-5
3.70 x 10-5
Select
0.74 x 10
3.70 x 10-
Then using the amount
of MnO4", in moles, and
Mass of Fe+ (g) in 25
cm sample:
0.74 x 10-4
the molar ratio, select
1.85 x 10-4
the correct value for the
amount of Fe?+ ions, in
moles.
Mass of Fe+ (g) per iron
tablet:
Compare titration
results with iron content
on product label:
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

