A CHM 125 student performed the "Redox Titration" procedure as given in the laboratory manual. In this experiment, the titrant solution of potassium permanganate (KMNO4) was used to determine the percentage of sodium oxalate (Na,C2O4) in an unknown sample. The net ionic equation for the titration carried out was: 2MNO4 (aq) + 5C207(aq) + 16H*(aq) → 2Mn²*(aq) + 10CO2(g) + 8H2O(1) The data provided in the table below was acquired. Complete the table and determine the percent of sodium oxalate in the unknown sample based on this one titration. Molarity of KMNO4 Titrant Solution: 0.0203 M Trial 1 Weight of Oxalate Unknown 0.954 g Starting Buret Reading: 0.65 mL 197 Ending Buret Reading: 34.85 mL permanganate s order to use 35.00 mL of Titrant Used: mmol of MnO4 mmol of C20, Weight of NazC204 in Unknown: Percent of NazC204 in Unknown:
A CHM 125 student performed the "Redox Titration" procedure as given in the laboratory manual. In this experiment, the titrant solution of potassium permanganate (KMNO4) was used to determine the percentage of sodium oxalate (Na,C2O4) in an unknown sample. The net ionic equation for the titration carried out was: 2MNO4 (aq) + 5C207(aq) + 16H*(aq) → 2Mn²*(aq) + 10CO2(g) + 8H2O(1) The data provided in the table below was acquired. Complete the table and determine the percent of sodium oxalate in the unknown sample based on this one titration. Molarity of KMNO4 Titrant Solution: 0.0203 M Trial 1 Weight of Oxalate Unknown 0.954 g Starting Buret Reading: 0.65 mL 197 Ending Buret Reading: 34.85 mL permanganate s order to use 35.00 mL of Titrant Used: mmol of MnO4 mmol of C20, Weight of NazC204 in Unknown: Percent of NazC204 in Unknown:
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.103QE
Related questions
Question
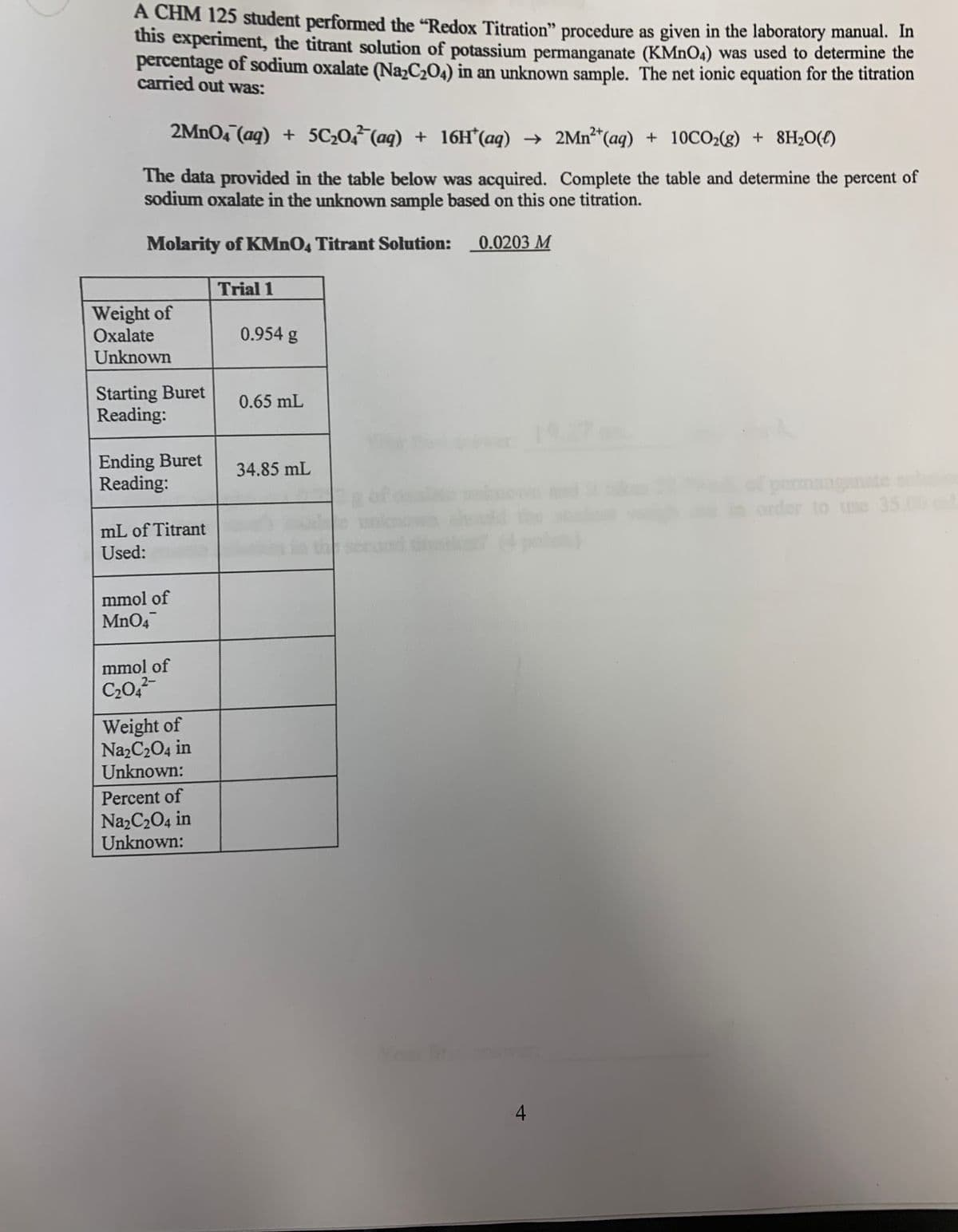
Transcribed Image Text:A CHM 125 student performed the "Redox Titration" procedure as given in the laboratory manual. In
this experiment, the titrant solution of potassium permanganate (KMNO4) was used to determine the
percentage of sodium oxalate (NazC2O4) in an unknown sample. The net ionic equation for the titration
carried out was:
2MnO4 (aq) + 5C20,7(aq) + 16H*(aq) → 2MN²*(aq) + 10CO2(g) + 8H2O(£)
The data provided in the table below was acquired. Complete the table and determine the percent of
sodium oxalate in the unknown sample based on this one titration.
Molarity of KMNO, Titrant Solution:
0.0203 M
Trial 1
Weight of
Oxalate
0.954 g
Unknown
Starting Buret
Reading:
0.65 mL
Ending Buret
Reading:
34.85 mL
of permanganate sl
order to use 35.00
mL of Titrant
Used:
mmol of
MnO4
mmol of
2-
Weight of
NazC204 in
Unknown:
Percent of
Na,C204 in
Unknown:
You wer
4-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning