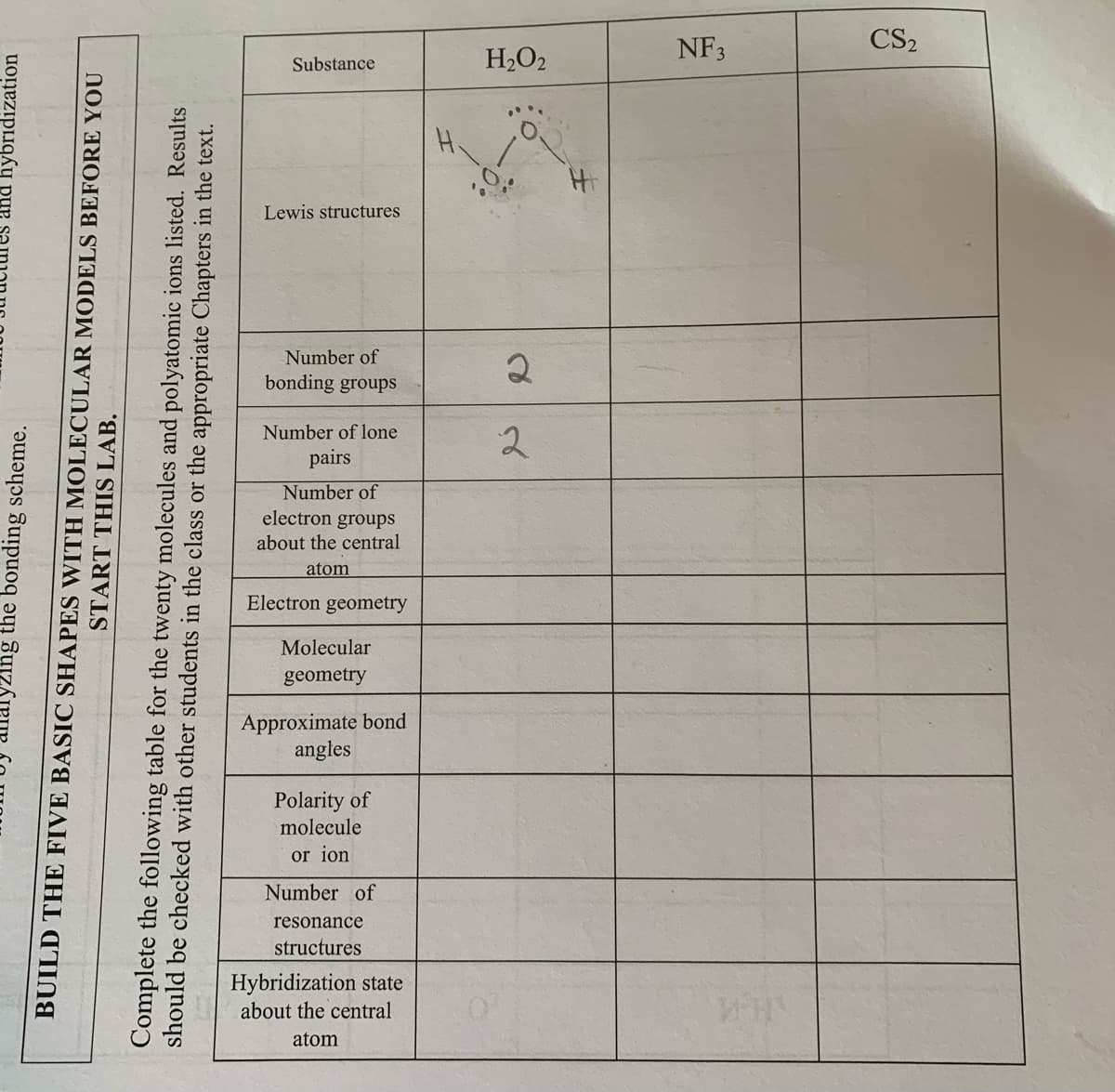
NF3 CS2 Substance H2O2 Lewis structures Number of bonding groups Number of lone 2 pairs Number of electron groups about the central atom Electron geometry Molecular geometry Approximate bond angles Polarity of molecule or ion Number of resonance structures Hybridization state about the central atom
NF3 CS2 Substance H2O2 Lewis structures Number of bonding groups Number of lone 2 pairs Number of electron groups about the central atom Electron geometry Molecular geometry Approximate bond angles Polarity of molecule or ion Number of resonance structures Hybridization state about the central atom
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU2: Smells: Molecular Structure And Properties
SectionU2.11: Let's Build It: Molecular Shape
Problem 3E
Related questions
Question
CS2?
Transcribed Image Text:NF3
CS2
H,O2
hybridization
lalyzing the bonding scheme.
BUILD THE FIVE BASIC SHAPES WITH MOLECULAR MODELS BEFORE YOU
START THIS LAB.
Complete the following table for the twenty molecules and polyatomic ions listed. Results
should be checked with other students in the class or the appropriate Chapters in the text.
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning