ng poin ave the same melting point nternuclear distance is smaller; therefore, the force is stronger and come. charge is higher; therefore, interactions are stronger and require mor y have the same charge and internuclear distance; therefore, interacti ength and require the same amount of energy to overcome. and 5 and 5 2 and 5 MacBook Air 000
ng poin ave the same melting point nternuclear distance is smaller; therefore, the force is stronger and come. charge is higher; therefore, interactions are stronger and require mor y have the same charge and internuclear distance; therefore, interacti ength and require the same amount of energy to overcome. and 5 and 5 2 and 5 MacBook Air 000
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter7: Chemical Bonding And Molecular Structure
Section: Chapter Questions
Problem 7.13PAE: 7.13 Figure 7-2 depicts the interactions of an ion with its first nearest neighbors, second nearest...
Related questions
Question
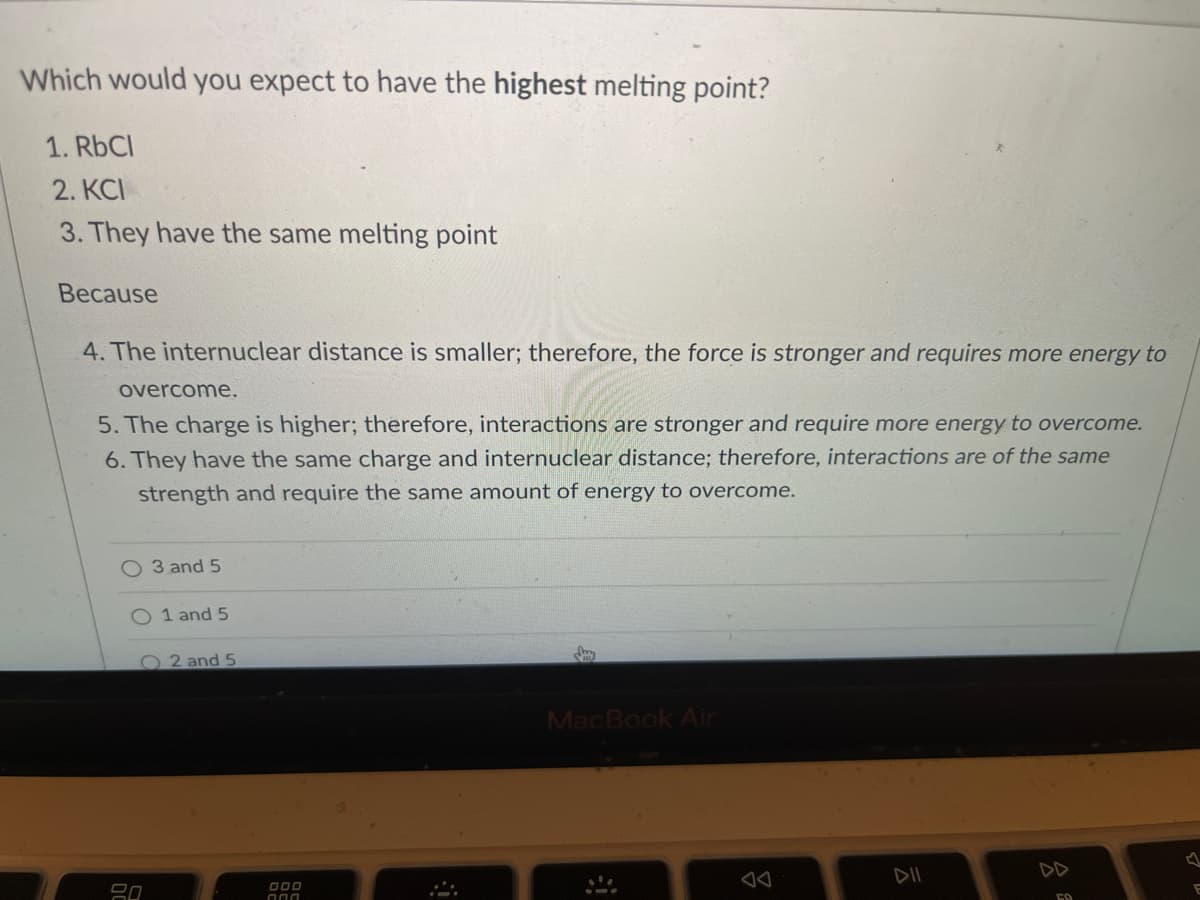
Transcribed Image Text:Which would you expect to have the highest melting point?
1. RbCl
2. KCI
3. They have the same melting point
Because
4. The internuclear distance is smaller; therefore, the force is stronger and requires more energy to
overcome.
5. The charge is higher; therefore, interactions are stronger and require more energy to overcome.
6. They have the same charge and internuclear distance; therefore, interactions are of the same
strength and require the same amount of energy to overcome.
O 3 and 5
O 1 and 5
2 and 5
MacBook Air
DII
DD
O00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
