TABLE 2.5 Important properties of liquid water compared with those of n-pentane, a nonpolar, nonhydrogen-bonding liquid Property Water n-Pentane Molecular weight (g/mol) 18.02 72.15 Density (g/cm) 0.997 0.626 Boiling point (°C) 100 36.1 Dielectric constant 78.3 1.84 Viscosity (g/cm - s) Surface tension (dyne/cm) 0.890 x 10-2 0.228 x 10-2 71.97 17
TABLE 2.5 Important properties of liquid water compared with those of n-pentane, a nonpolar, nonhydrogen-bonding liquid Property Water n-Pentane Molecular weight (g/mol) 18.02 72.15 Density (g/cm) 0.997 0.626 Boiling point (°C) 100 36.1 Dielectric constant 78.3 1.84 Viscosity (g/cm - s) Surface tension (dyne/cm) 0.890 x 10-2 0.228 x 10-2 71.97 17
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section1.6: Physical Properties
Problem 2RC: 2. A piece of a polypropylene rope (used for water skiing) floats on water, whereas a terephthalate...
Related questions
Question
Suppose a chloride ion and a sodium ion are separated by a center–center
distance of 5 Å. Is the interaction energy (the energy required to pull them
infinitely far apart) predicted to be larger if the medium between them is
water, or if it is n-pentane? (as shown)
If Ca2+, Na+, and F- each have ionic radii ~1.16. Which ionic bond is stronger: Ca-F or Na-F?
If Ca2+ is often bound on the surface of a protein by carboxylic acid functional groups. If the pKa of a particular -COOH group is 4.2, would
you predict Ca2+ to be most tightly bound at pH 8, pH 4.2, or pH 3? Explain your answer.
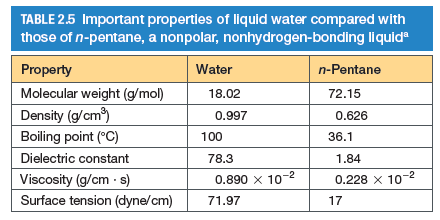
Transcribed Image Text:TABLE 2.5 Important properties of liquid water compared with
those of n-pentane, a nonpolar, nonhydrogen-bonding liquid
Property
Water
n-Pentane
Molecular weight (g/mol)
18.02
72.15
Density (g/cm)
0.997
0.626
Boiling point (°C)
100
36.1
Dielectric constant
78.3
1.84
Viscosity (g/cm - s)
Surface tension (dyne/cm)
0.890 x 10-2
0.228 x 10-2
71.97
17
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning