Nitrite concentration is commonly determined by interpolation method using spectrophotometry (an instrumental method) by a reaction called Griess Reaction. In this reaction, the sample containing nitrite is reacted with sulfanilamide and N-(1- Napthyl) ethylenediamine to form a colored species that absorbs at 550 nm. Using automated flow analysis instrument, the following results were obtained for the standard solutions of nitrite and for a sample containing an unknown amount. Concentration of Standard Solutions (x-axis) 2.000 UM 6.000 UM 10.00 UM 14.00 UM 18.00 UM Unknown Instrument Response (y-axis) 0.065 0.205 0.338 0.474 0.598 0.186 The instrument response (y-axis, absorbance at 550 nm) is plotted against standard concentration (x-axis, concentration) to obtain the calibration curve. Access the calibration curve using this link (open in a new window). Linear regression analysis was used to obtain the regression equation (y = mx + b) and found out to be y = 0.03338 x + 0.00225. Calculate the concentration of nitrite the unknown sample using the regression equation. Express your answers in 4 significant figures.
Nitrite concentration is commonly determined by interpolation method using spectrophotometry (an instrumental method) by a reaction called Griess Reaction. In this reaction, the sample containing nitrite is reacted with sulfanilamide and N-(1- Napthyl) ethylenediamine to form a colored species that absorbs at 550 nm. Using automated flow analysis instrument, the following results were obtained for the standard solutions of nitrite and for a sample containing an unknown amount. Concentration of Standard Solutions (x-axis) 2.000 UM 6.000 UM 10.00 UM 14.00 UM 18.00 UM Unknown Instrument Response (y-axis) 0.065 0.205 0.338 0.474 0.598 0.186 The instrument response (y-axis, absorbance at 550 nm) is plotted against standard concentration (x-axis, concentration) to obtain the calibration curve. Access the calibration curve using this link (open in a new window). Linear regression analysis was used to obtain the regression equation (y = mx + b) and found out to be y = 0.03338 x + 0.00225. Calculate the concentration of nitrite the unknown sample using the regression equation. Express your answers in 4 significant figures.
Chapter12: Spectrochemical Methods
Section: Chapter Questions
Problem 7P
Related questions
Question
Please help me with this
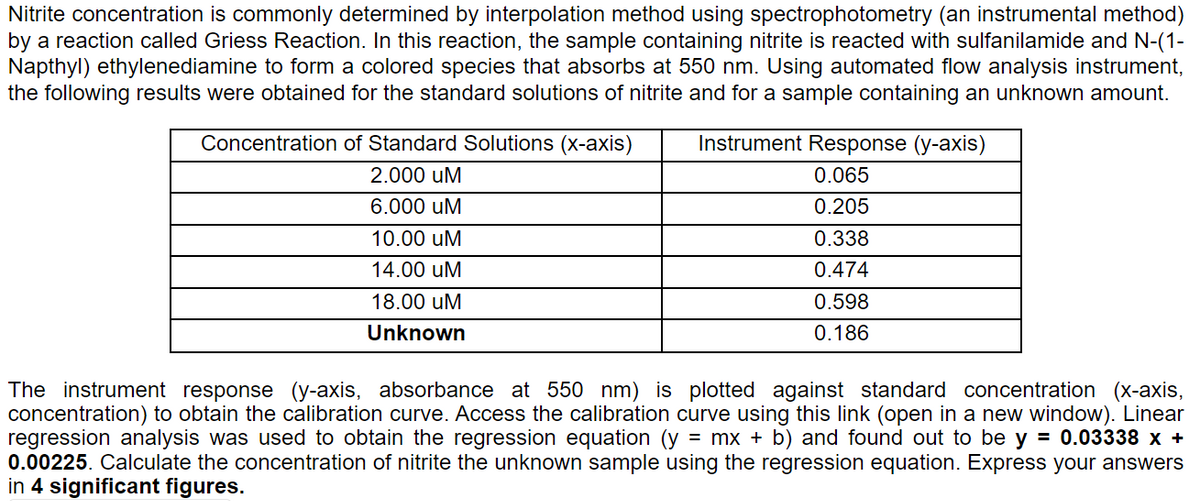
Transcribed Image Text:Nitrite concentration is commonly determined by interpolation method using spectrophotometry (an instrumental method)
by a reaction called Griess Reaction. In this reaction, the sample containing nitrite is reacted with sulfanilamide and N-(1-
Napthyl) ethylenediamine to form a colored species that absorbs at 550 nm. Using automated flow analysis instrument,
the following results were obtained for the standard solutions of nitrite and for a sample containing an unknown amount.
Concentration of Standard Solutions (x-axis)
2.000 UM
6.000 UM
10.00 uM
14.00 uM
18.00 uM
Unknown
Instrument Response (y-axis)
0.065
0.205
0.338
0.474
0.598
0.186
The instrument response (y-axis, absorbance at 550 nm) is plotted against standard concentration (x-axis,
concentration) to obtain the calibration curve. Access the calibration curve using this link (open in a new window). Linear
regression analysis was used to obtain the regression equation (y = mx + b) and found out to be y = 0.03338 x +
0.00225. Calculate the concentration of nitrite the unknown sample using the regression equation. Express your answers
in 4 significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning