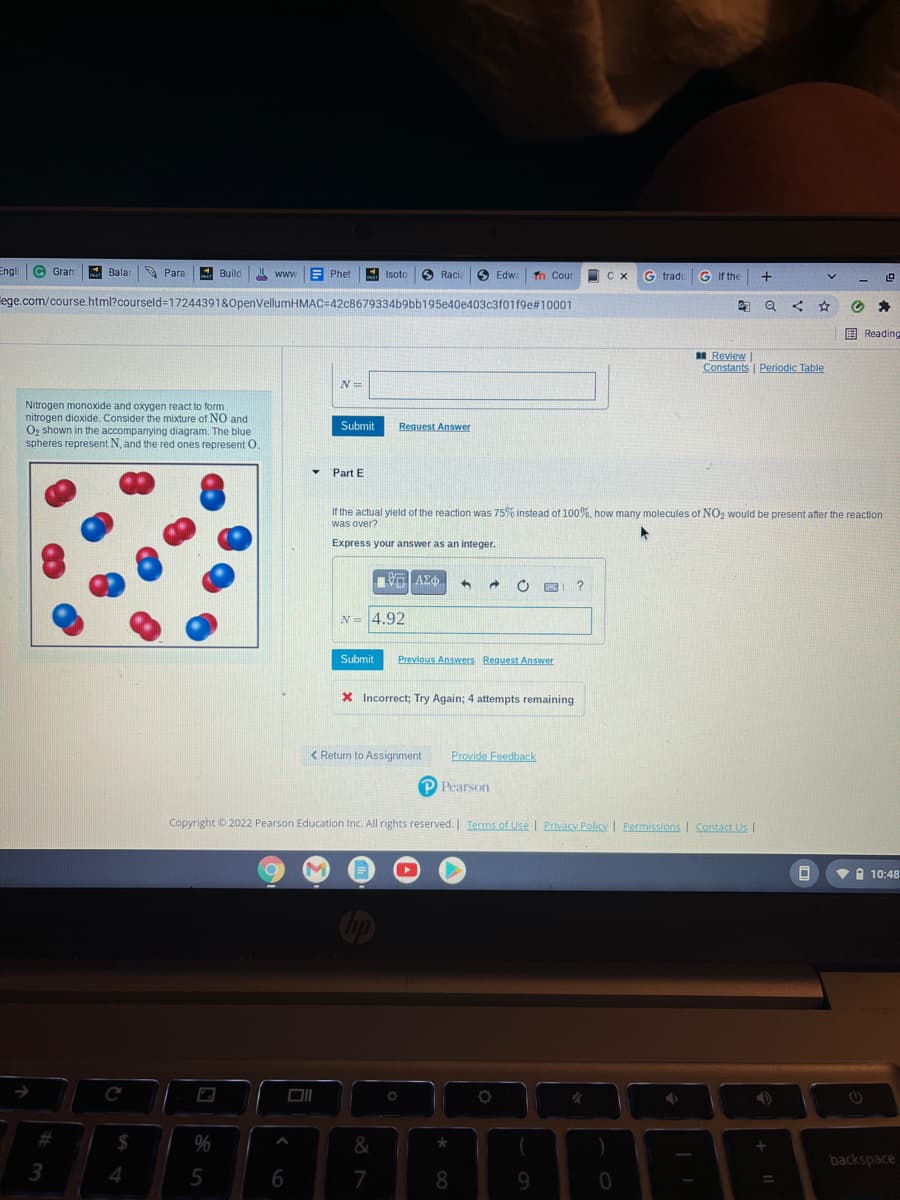
Nitrogen monoxide and oxygen react to form nitrogen dioxide. Consider the mixture of NO and Og shown in the accompanying diagram. The blue spheres represent N, and the red ones represent O. Submit Reguest Answer Part E If the actual yield of the reaction was 75% instead of 100%, how many molecules of NO2 would be present after the reaction was over? Express your answer as an integer. N= 4.92 Submit Previous Answers Request Answer
Nitrogen monoxide and oxygen react to form nitrogen dioxide. Consider the mixture of NO and Og shown in the accompanying diagram. The blue spheres represent N, and the red ones represent O. Submit Reguest Answer Part E If the actual yield of the reaction was 75% instead of 100%, how many molecules of NO2 would be present after the reaction was over? Express your answer as an integer. N= 4.92 Submit Previous Answers Request Answer
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
100%
Transcribed Image Text:Engli
G Gran
1 Balar
A Para
E Phet
A Isoto
Edwa
Build
O Racia
O
G tradu
www
Tn Cour
G If the
lege.com/course.html?courseld%317244391&0penVellumHMAC=42c8679334b9bb195e40e403c3f01f9e#10001
Q < *
国 Reading
I Review |
Constants | Periodic Table
N =
Nitrogen monoxide and oxygen react to form
nitrogen dioxide. Consider the mixture of NO and
O2 shown in the accompanying diagram. The blue
spheres represent N, and the red ones represent O
Submit
Request Answer
Part E
If the actual yield of the reaction was 75% instead of 100%, how many molecules of NO2 would be present after the reaction
was over?
Express your answer as an integer.
回?
N= 4.92
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 4 attempts remaining
< Return to Assignment
Provide Feedback
P Pearson
Copyright 2022 Pearson Education Inc. All rights reserved. | Terms of Use Privacy Policy | Permissions | Contact Us |
V I 10:48
%23
2$
&
backspace
4.
5
6
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



