Nitrous acid has a K, of 4.5 x 10 4. Part A What is the pH of a buffer solution containing 0.19 M HNO2 and 0.12 M NO, ? Express your answer using two decimal places. V ΑΣφ pH = Submit Request Answer
Nitrous acid has a K, of 4.5 x 10 4. Part A What is the pH of a buffer solution containing 0.19 M HNO2 and 0.12 M NO, ? Express your answer using two decimal places. V ΑΣφ pH = Submit Request Answer
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 29Q: Give three example solutions that fit each of the following descriptions. a. a strong electrolyte...
Related questions
Question
Do it all please neat and clean...
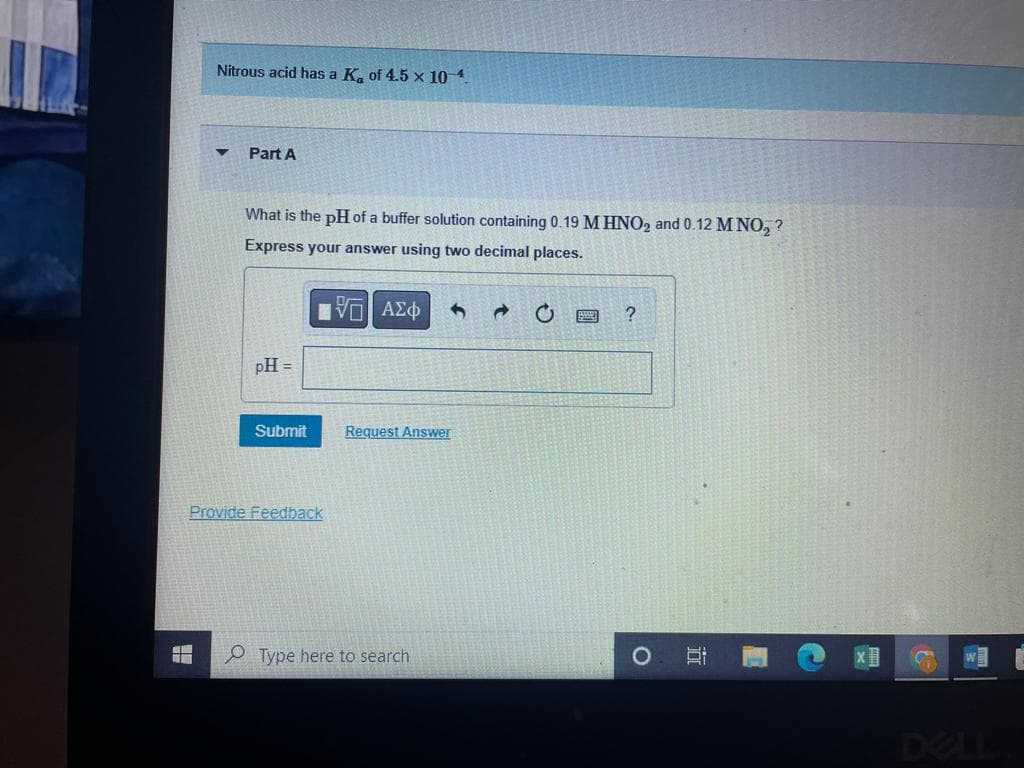
Transcribed Image Text:Nitrous acid has a K, of 4.5 x 10 4
Part A
What is the pH of a buffer solution containing 0.19 M HNO2 and 0.12 M NO, ?
Express your answer using two decimal places.
pH =
Submit
Request Answer
Provide Feedback
Type here to search
DELL
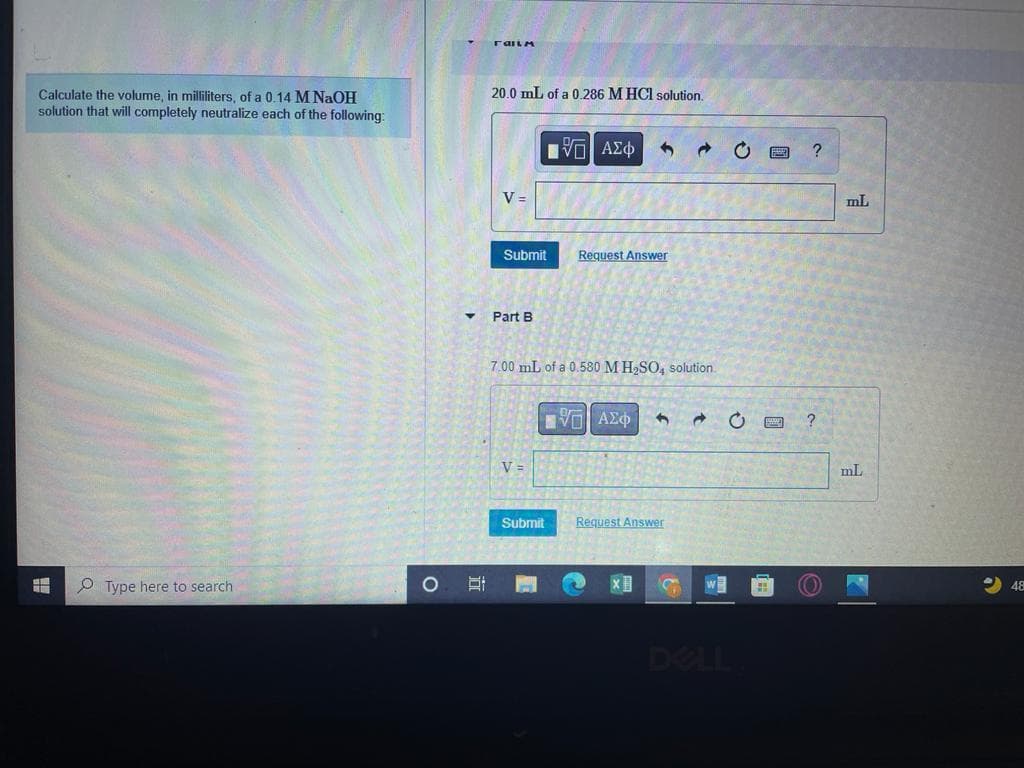
Transcribed Image Text:20.0 mL of a 0,286 M HCI solution.
Calculate the volume, in milliliters, of a 0.14 M NaOH
solution that will completely neutralize each of the following:
Eνα ΑΣφ
V =
mL
Submit
Request Answer
Part B
7.00 mL of a 0.580 M H,SO, solution.
V =
Submit
Request Answer
O Type here to search
WE
48
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning