Based only on the half-reactions in table below, determine what spontaneous reaction will occur if the following substances are mixed together. Include physical states in your answer.
Based only on the half-reactions in table below, determine what spontaneous reaction will occur if the following substances are mixed together. Include physical states in your answer.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter18: Nuclear Reactions
Section: Chapter Questions
Problem 19QAP: Balance the following equations by filling in the blanks. (a) 92235U+01n54137_+201n+_ (b)...
Related questions
Question
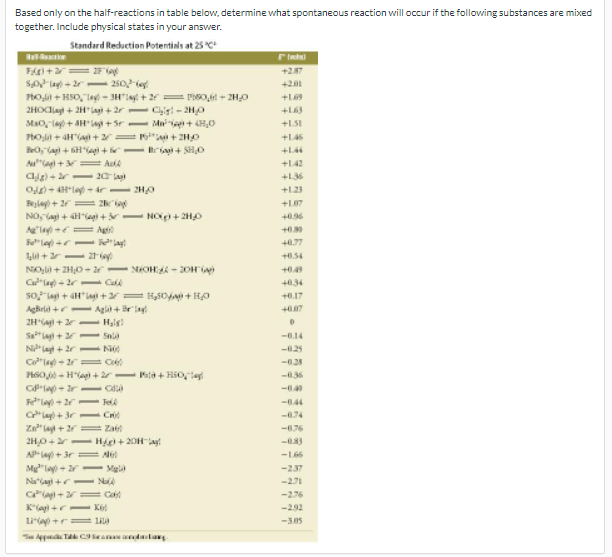
Transcribed Image Text:Based only on the half-reactions in table below, determine what spontaneous reaction will occur if the following substances are mixed
together. Include physical states in your ansver.
Standard Reduction Potentials at 25 C*
+287
Solag + 2r- 250ie
Ptoi) + HSO, lay - 3H"Iay + 2 = lb80,il + ZH,o
2HOdlayi + 2H*lai + 2r - Cisi - 2H0
+201
+L63
Mao, lag + AHagi + Sr
+1SI
Ptol + H"ag + = i + 2H0
+L46
Bria + SHO
+L4
Au"agi += AuA
Cg) + r- 20 lag
O+ AH*lay - ár- 2HO
+142
+136
+123
ylay+ 2r 2io
NO, agi +Hag) + - NOg + 2H0
lag = A
F"lay +r- lay
+L07
+0.96
+0.30
+0.77
+0.54
NOl+ 2H,0- ar - NOH - 20H
+0.49
+034
+0.17
Agkrla +r-Agli + Briay
2Ha + - Hais!
Salag + - Snia
N Layi + 2r- Nộ
Co"lag - 2= Co
PHSO j - Hagi + 2r- Pata + Hio,e
Clay - 2r- Cdla
lay + 2r FelA
Clay) + 3r- Cri
Za"lag + 2r = Za
2H,0+2 -Higi + 20Hay
AP-leg+ 3r= N
Mg"lay - 2r
Naagi +r- NA
Cagi+ 2= Ca
Kagi +r- Ke
Lia +r= lila
-0.14
-025
-028
-036
-0.40
-0.44
-0.74
-0.76
-L66
Mgl
-237
-271
-2.76
-292
-305
S Aepn T C9 Sana dla

Transcribed Image Text:I3 l'and Fe", Fe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning