Now you get to balance this equation (answer in the same way as in the problem above): NO2(g) + OH (aq) – NO3-(aq) + NO, (aq) + H20(1) Don't forget to put in coefficients of one and to use the lowest possible whole number coefficients.
Now you get to balance this equation (answer in the same way as in the problem above): NO2(g) + OH (aq) – NO3-(aq) + NO, (aq) + H20(1) Don't forget to put in coefficients of one and to use the lowest possible whole number coefficients.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.71PAE: 4.71 The particulate scale drawing shown depict the products of a reaction between N2 and O2...
Related questions
Question
100%
Balancing redox. I am not even sure what the half reactions would look like in this.
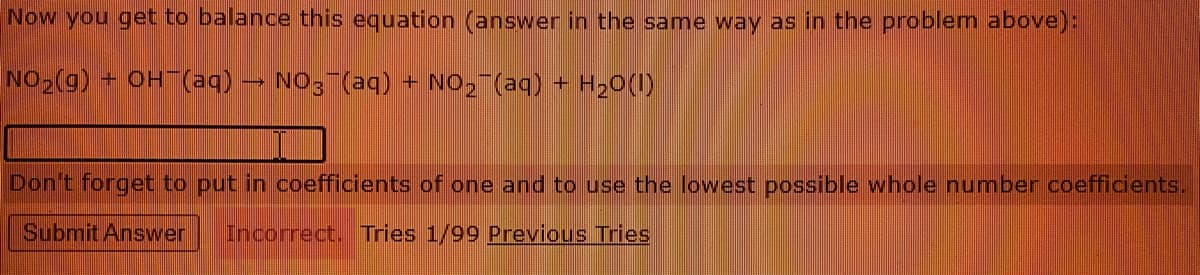
Transcribed Image Text:Now you get to balance this equation (answer in the same way as in the problem above):
NO2(g) OH (aq)
NO3 (aq) + NO2¯(aq) + H20(1)
Don't forget to put in coefficients of one and to use the lowest possible whole number coefficients.
Submit Answer
Incorrect. Tries 1/99 Previous Tries
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning