Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 6ALQ: onsider separate aqueous solutions of HCI and H2S04 with the same concentrations in terms of...
Related questions
Question
100%
7
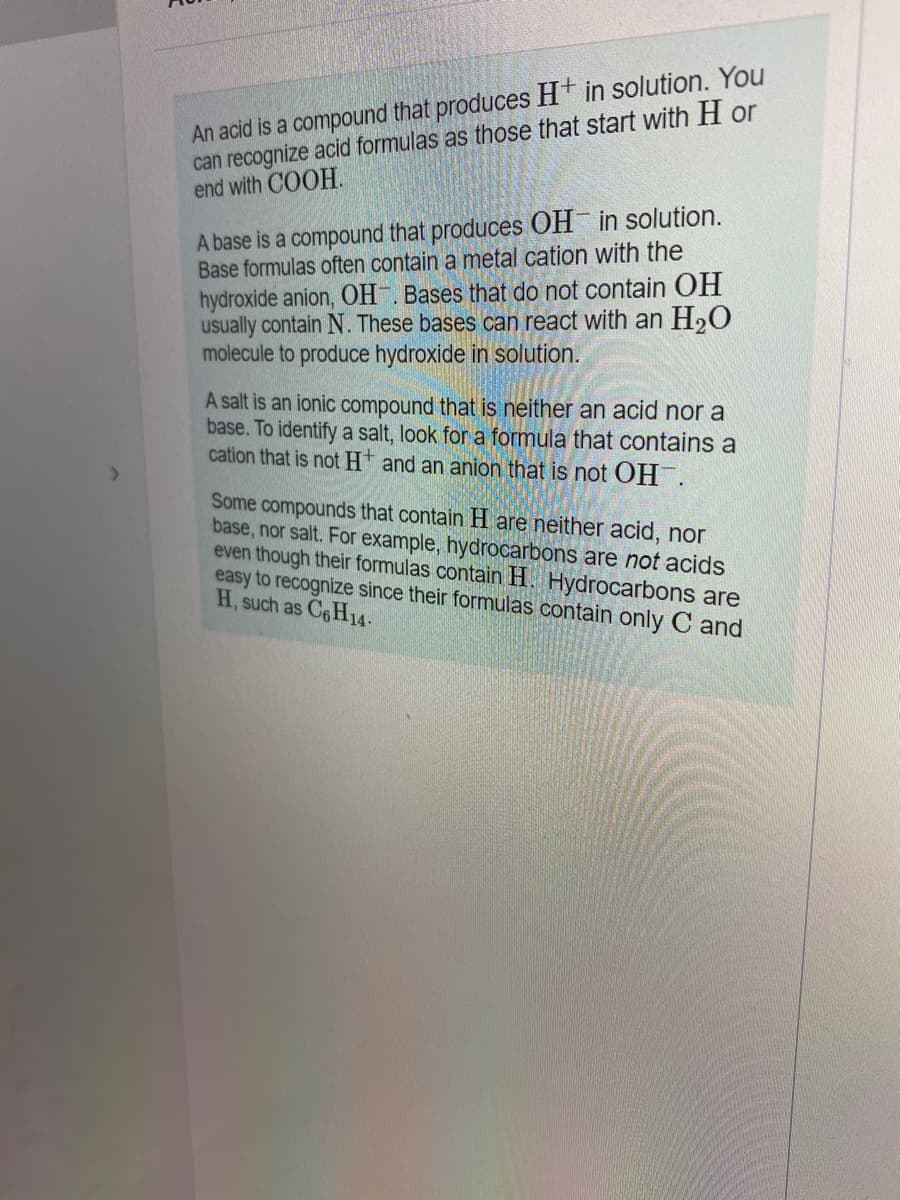
Transcribed Image Text:An acid is a compound that produces H* in solution. You
can recognize acid formulas as those that start with H or
end with COOH.
A base is a compound that produces OH in solution.
Base formulas often contain a metal cation with the
hydroxide anion, OH . Bases that do not contain OH
usually contain N. These bases can react with an H20
molecule to produce hydroxide in solution.
A salt is an ionic compound that is neither an acid nor a
base. To identify a salt, look for a formula that contains a
cation that is not Ht and an anion that is not OH.
Some compounds that contain H are neither acid, nor
base, nor salt. For example, hydrocarbons are not acids
even though their formulas contain H. Hydrocarbons are
easy to recognize since their formulas contain only C and
H, such as C6H14.
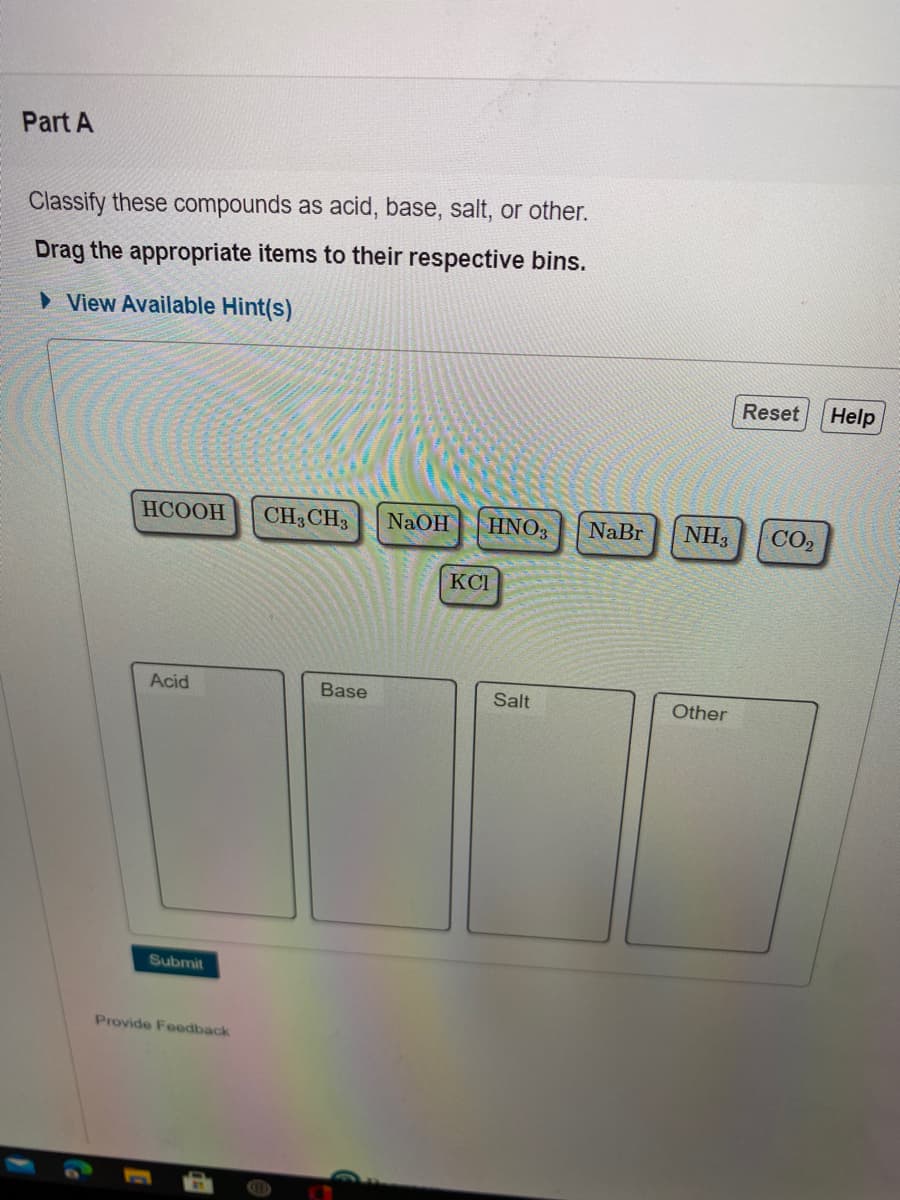
Transcribed Image Text:Part A
Classify these compounds as acid, base, salt, or other.
Drag the appropriate items to their respective bins.
» View Available Hint(s)
Reset
Help
НСООН
CH3 CH3
NaOH
HNO3
NaBr
NH3
CO2
KCI
Acid
Base
Salt
Other
Submit
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning