nt BACK PRINTER VERSION FULL SCREEN CALCULATOR SPECTROSCOPY AND REACTIVITY TO4/S06 The energy levels the electron can occupy in the Bet ion can be calculated using the energy level equation. A Be emitted a photon with a frequency of 9.86 x 105 Hz. What is the value of the level n for the new energy? ion with an electron in the n 4.00 level Speed of Light c 3.00x108 m/s Planck's Constant h 6.63x1034 J S Rydberg's Constant RH=2.18x1o-18J Avogadro's No. NA 6.02x1023mol1 Energy Level Eqn En(J)-RH(Z2/n2) Z=nuclear charge =quantum number exact number, no tolerance
nt BACK PRINTER VERSION FULL SCREEN CALCULATOR SPECTROSCOPY AND REACTIVITY TO4/S06 The energy levels the electron can occupy in the Bet ion can be calculated using the energy level equation. A Be emitted a photon with a frequency of 9.86 x 105 Hz. What is the value of the level n for the new energy? ion with an electron in the n 4.00 level Speed of Light c 3.00x108 m/s Planck's Constant h 6.63x1034 J S Rydberg's Constant RH=2.18x1o-18J Avogadro's No. NA 6.02x1023mol1 Energy Level Eqn En(J)-RH(Z2/n2) Z=nuclear charge =quantum number exact number, no tolerance
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 80QAP: In the photoelectric effect, electrons are ejected from a metal surface when light strikes it. A...
Related questions
Question
Transcribed Image Text:nt
BACK
PRINTER VERSION
FULL SCREEN
CALCULATOR
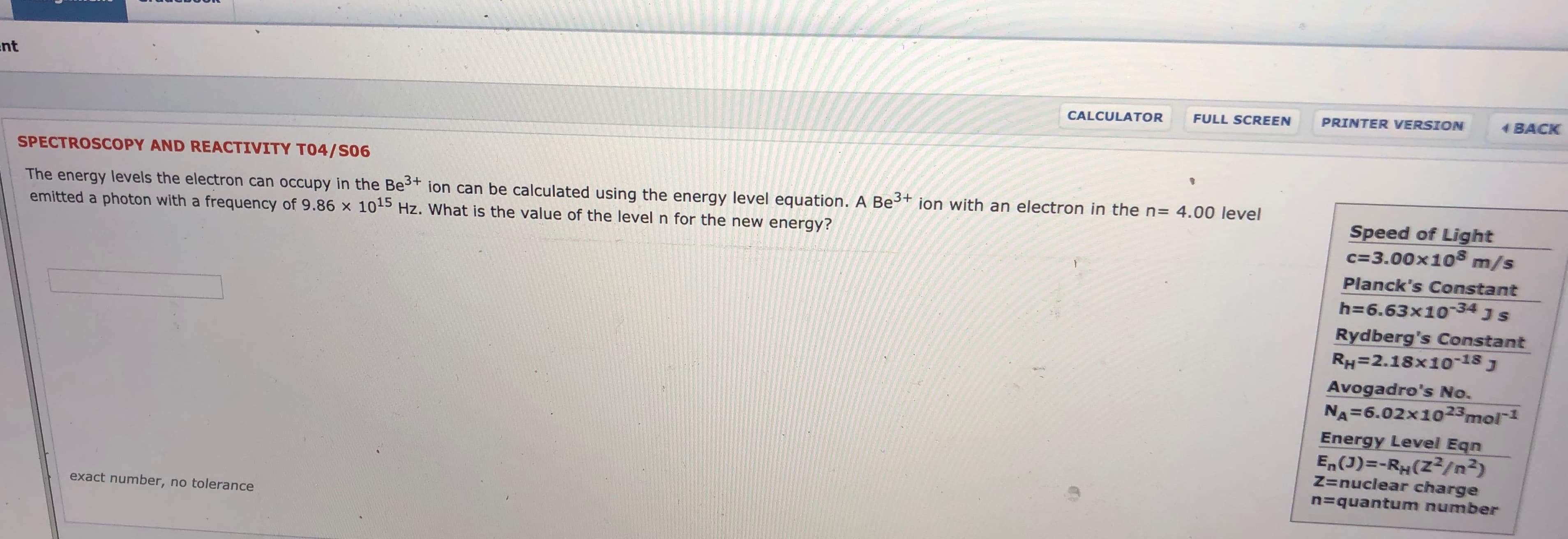
SPECTROSCOPY AND REACTIVITY TO4/S06
The energy levels the electron can occupy in the Bet ion can be calculated using the energy level equation. A Be
emitted a photon with a frequency of 9.86 x 105 Hz. What is the value of the level n for the new energy?
ion with an electron in the n
4.00 level
Speed of Light
c 3.00x108
m/s
Planck's Constant
h 6.63x1034 J S
Rydberg's Constant
RH=2.18x1o-18J
Avogadro's No.
NA 6.02x1023mol1
Energy Level Eqn
En(J)-RH(Z2/n2)
Z=nuclear charge
=quantum number
exact number, no tolerance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning