O Chemical Reactions Dilution 3/5 [ A chemist must prepare 125. mL of 180. mM aqueous copper(II) sulfate (CuSO4) working solution. He'll do this by pouring out some 0.198 mol aqueous L copper(II) sulfate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the copper(II) sulfate stock solution that the chemist should pour out. Round your answer to 3 significant digits. Oml. G
O Chemical Reactions Dilution 3/5 [ A chemist must prepare 125. mL of 180. mM aqueous copper(II) sulfate (CuSO4) working solution. He'll do this by pouring out some 0.198 mol aqueous L copper(II) sulfate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the copper(II) sulfate stock solution that the chemist should pour out. Round your answer to 3 significant digits. Oml. G
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 98AP
Related questions
Question
100%
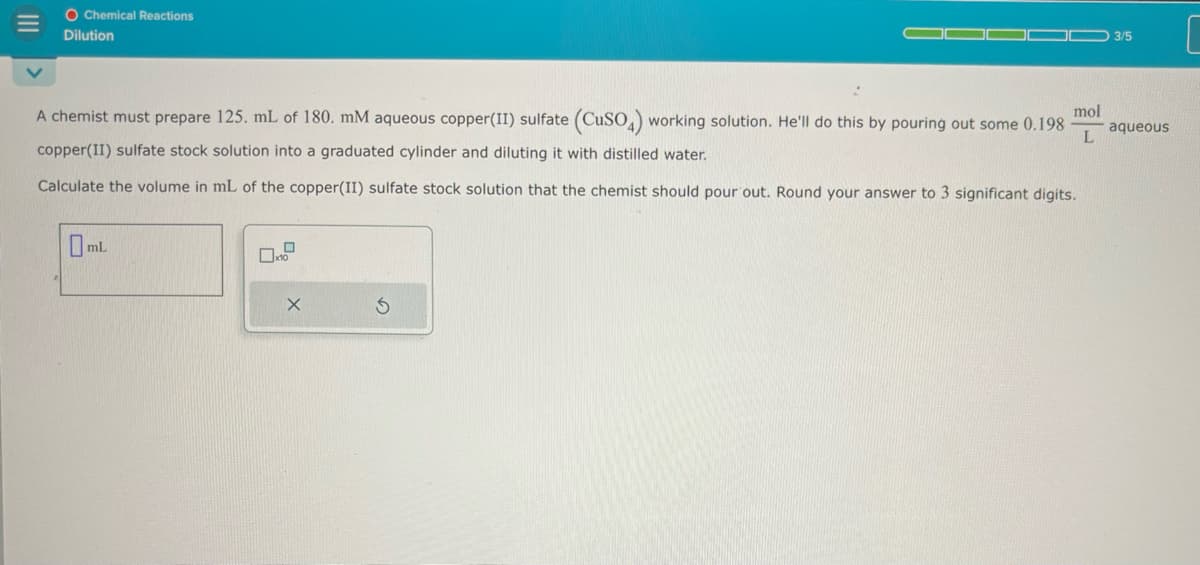
Transcribed Image Text:O Chemical Reactions
Dilution
3/5
[
A chemist must prepare 125. mL of 180. mM aqueous copper(II) sulfate (CuSO4) working solution. He'll do this by pouring out some 0.198
mol
aqueous
L
copper(II) sulfate stock solution into a graduated cylinder and diluting it with distilled water.
Calculate the volume in mL of the copper(II) sulfate stock solution that the chemist should pour out. Round your answer to 3 significant digits.
Oml.
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning