O CHEMICAL REACTIONS Identifying precipitation, combustion and acid-base reactions Classify each chemical reaction: type of reaction (check all that apply) reaction O combination O precipitation 2CH, (CH,),CH, (g) + 130, (g) - 8co,(s) + 10H,0 (g) O single replacement O combustion O double replacement O decomposition acid-base O combination O precipitation O single replacement O double replacement O combustion H (аq) + NaOH (aq) Nal (aq) + H,0(1) O acid-base O decomposition O combination O precipitation O single replacement O double replacement O decomposition O combustion K Br (aq) + AgNO, (aq) → KNO, (ag) + Ag Br(s) O acid-base O combination O precipitation O single replacement O double replacement O decomposition O combustion кон (аq) + нBrо, (ag) - к вго, (аaq) + н,о() O acid-base
O CHEMICAL REACTIONS Identifying precipitation, combustion and acid-base reactions Classify each chemical reaction: type of reaction (check all that apply) reaction O combination O precipitation 2CH, (CH,),CH, (g) + 130, (g) - 8co,(s) + 10H,0 (g) O single replacement O combustion O double replacement O decomposition acid-base O combination O precipitation O single replacement O double replacement O combustion H (аq) + NaOH (aq) Nal (aq) + H,0(1) O acid-base O decomposition O combination O precipitation O single replacement O double replacement O decomposition O combustion K Br (aq) + AgNO, (aq) → KNO, (ag) + Ag Br(s) O acid-base O combination O precipitation O single replacement O double replacement O decomposition O combustion кон (аq) + нBrо, (ag) - к вго, (аaq) + н,о() O acid-base
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 26QAP: Consider the following generic equation OH(aq)+HB(aq) B(aq)+H2OFor which of the following pairs...
Related questions
Question
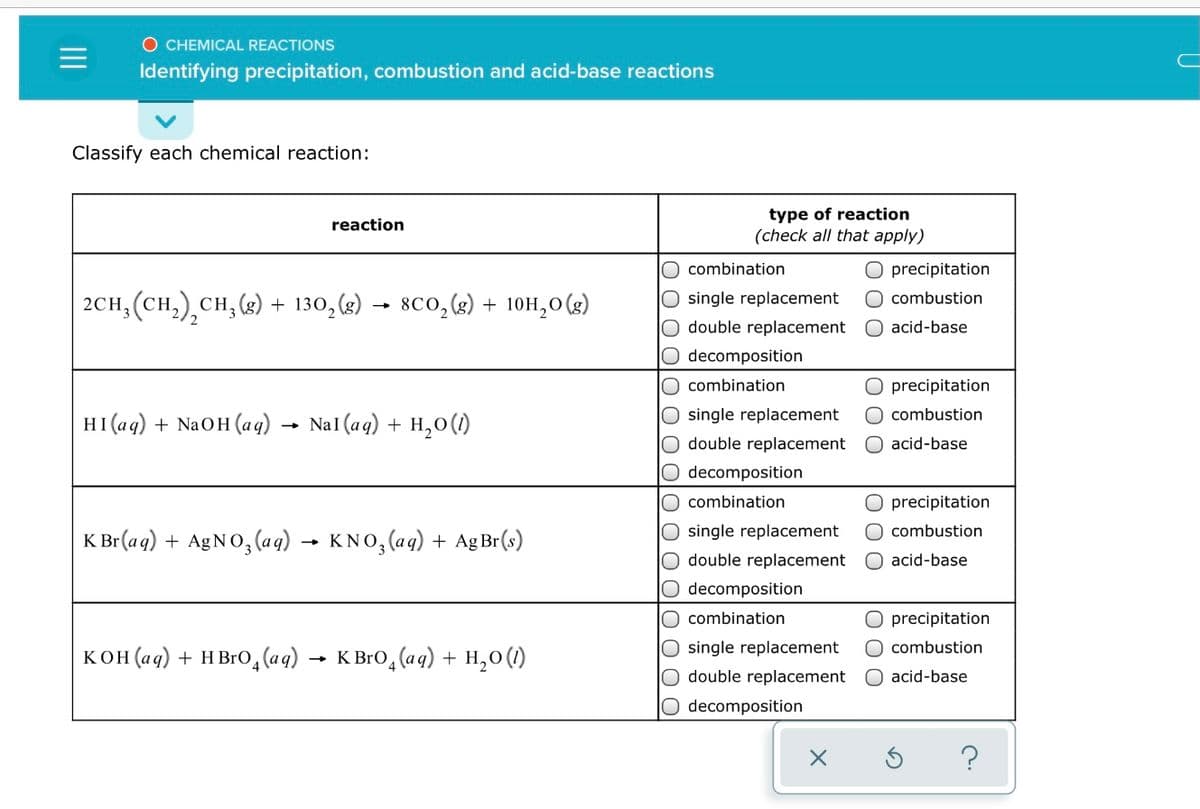
Transcribed Image Text:O CHEMICAL REACTIONS
Identifying precipitation, combustion and acid-base reactions
Classify each chemical reaction:
type of reaction
(check all that apply)
reaction
combination
O precipitation
2CH, (CH,),CH, (3)
+ 130, (2) → 8Co, (s) + 10H,0 (g)
O combustion
single replacement
double replacement O acid-base
decomposition
combination
O precipitation
single replacement
O combustion
HI(aq) + NaOH (aq)
NaI (aq) + H,0(1)
double replacement
O acid-base
O decomposition
combination
precipitation
K Br (a q) + AGNO, (ag) → KNO, (aq) + AgBr(s)
O single replacement
combustion
double replacement
acid-base
decomposition
combination
precipitation
single replacement
combustion
кон(аq) + нвю, (aq) — к вго, (аq) + н,о()
double replacement
acid-base
decomposition
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning