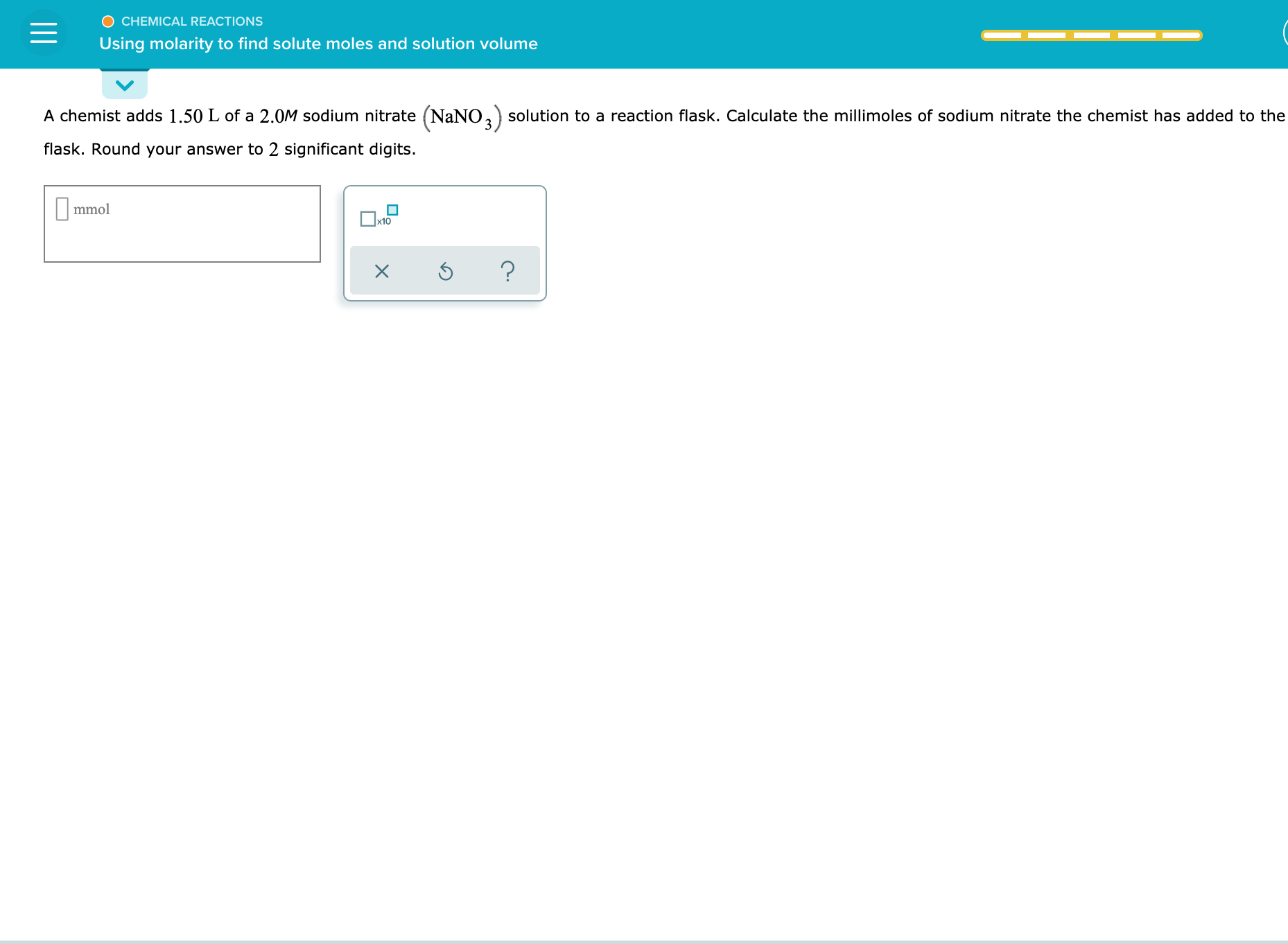
O CHEMICAL REACTIONS Uusing molarity to find solute moles and solution volume A chemist adds 1.50 L of a 2.0M sodium nitrate (NaNO, solution to a reaction flask. Calculate the millimoles of sodium nitrate the chemist has added to the flask. Round your answer to 2 significant digits. ||mmol
Q: A chemist prepares a solution of aluminum chloride (AICI,) by measuring out 46. umol of aluminum…
A:
Q: Aqueous hydrobromic acid HBr will react with solid sodium hydroxide NaOH to produce aqueous sodium…
A: Limiting reagent is the reactant that limits the chemical reaction and determines the amount of the…
Q: Convert 4.58 * 103cm3to each unit.a. L b. mL c. gal d. qtThe m in the equation for density is in…
A: Conversion of one unit to another unit is done by multiplying or dividing the value that is being…
Q: A chemist prepares a solution of magnesium fluoride MgF2 by measuring out 0.0042μmol of magnesium…
A: Given that: Moles of Magnesium fluoride = 0.0042μmol Volume = 450 mL = 0.450L Concentration =?
Q: Sodium carbonate (Na2 CO3) is used to neutralize the sulfuric acid spill. How many kilograms of…
A: The reaction between sodium carbonate and sulfuric acid is as follows, The given mass of sulfuric…
Q: A chemist prepares a solution of silver nitrate (AgNO, by measuring out 0.556 kg of silver nitrate…
A:
Q: A chemist must prepare 0.700 L of 1.00 M aqueous zinc nitrate (Zn(NO,),) working solution. He'll do…
A: Given: The concentration of the stock solution of zinc nitrate, (M1) = 6.12 mol/L=6.12 M. The…
Q: Aqueous hydrobromic acid HBr will react with solid sodium hydroxide NaOH to produce aqueous sodium…
A:
Q: O CHEMICAL REACTIONS Using molarity to find solute mass and solution volume Brittney v A chemist…
A:
Q: A chemist prepares a solution of calcium bromide (CaBr,) by measuring out 0.667 kg of calcium…
A: A Chemist prepares a solution of calcium bromide(CaBr2) by measuring out 0.667kg of calcium bromide…
Q: A chemist prepares a solution of copper(II) sulfate (CuSO,) by weighing out 30.0 g of copper(II)…
A: Given, mass of CuSO4 = 30.0 g Volume = 300 mL = 0.3 L
Q: A chemist must prepare 125. mL of 865. mM aqueous barium chlorate (Ba(CIo,)) working solution. He'll…
A: Moles of both solutions will be same. M1 × V1 = M2 × V2
Q: A major component of gasoline is octane C8H18 . When liquid octane is burned in air it reacts with…
A: The burning of octane in presence of air is an example of a combustion reaction. Combustion…
Q: A chemist prepares a solution of calcium bromide (CaBr, ) by measuring out 30.2 umol of calcium…
A: Given ; Moles of CaBr2 = 30.2 micromol And volume of solution = 300 mL
Q: A chemist makes 320. mL of calcium sulfate (CaSO,) working solution by adding distilled water to…
A:
Q: A chemist prepares a solution of sodium carbonate (Na,CO,) by measuring out 0.301 µmol of sodium…
A: Given : Moles of Na2CO3 = 0.301 µmol And volume of solution = 200 mL
Q: Aqueous hydrochloric acid (HCI) will react with solid sodium hydroxide (NaOH) to produce aqueous…
A: HCl + NaOH -> NaCl +H2O Molar mass of HCl = 36.5 g/mol Molar mass of NaCl = 40 g/mol
Q: A chemist prepares a solution of zinc nitrate by measuring out 78. umol of zinc nitrate into a 100.…
A: Given: The number of micromoles of zinc nitrate present = 78 μmol The volume of the solution = 100…
Q: A chemist prepares a solution of silver(I) nitrate (AgNO3) by measuring out 389. μmol of silver(I)…
A:
Q: A chemist prepares a solution of magnesium fluoride (MgF,) by measuring out 0.0118 µmol of magnesium…
A:
Q: Methanol (CH4O) has a density of 0.791 gmLgmL and a molar mass of 32.05 gmolgmol. How many moles of…
A: Methanol is an alcohol with a functional group -OH and its molecular formula is CH3OH. The density…
Q: Determine the molarity of aluminum sulfate in the solution prepared by dissolving 15.00 grams of…
A: Molarity of a substance is measured as the solute moles mixed in a solution of 1L. It's expressed as…
Q: O CHEMICAL REACTIONS Using molarity to find solute mass and solution volume A chemist adds 390.0 mL…
A:
Q: A chemist prepares a solution of mercury(1) iodide (Hgl,) by measuring out 0.01l g of mercury)…
A:
Q: A chemist prepares a solution of magnesium fluoride (MgF,) by measuring out 0.00744 umol of…
A:
Q: Calculate the mass (in grams) of oxalic acid (H2C2O4) needed to make 100.0 mL of a 0.250 M solution.…
A:
Q: A chemist adds 145.0 mL of a 0.00260 mol/L calcium sulfate (CaSO.) solution to a reaction flask.…
A: Given volume = 145 ml Molarity = 0.00260 mol/litre Molarity = number of moles Volume in litre
Q: A chemist adds 410.0mL of a 4.8x10−5/mmolL mercury(II) iodide HgI2 solution to a reaction flask.…
A: Given, Volume of mercury (II) iodide = 410.0 mL { number of significant figure = 4}…
Q: A chemist prepares a solution of sodium carbonate (Na, CO,) by measuring out 77.3 g of sodium…
A: Given :- Mass of Na2CO3 = 77.3 g Volume of solution = 300. mL To calculate :- Molar concentration…
Q: Wine goes bad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas…
A: Formula Used No of moles = weight / molecular weight
Q: Calculating molárity ušing sóluté mcles A chemist prepares a solution of potassium permanganate…
A: Given : moles of KMnO4 = 15.2 micromol And volume of solution = 350 mL
Q: Aqueous hydrochloric acid (HCI) will react with solid sodium hydroxide (NAOH) to produce aqueous…
A: Given: The mass of HCl = 1.8 g The mass of NaOH = 1.16 g We have to calculate the mass of water…
Q: A chemist prepares a solution of silver(I) nitrate (AgNO3) by measuring out 78.μmol of silver(I)…
A: Molarity : molarity of a solute is the number of moles of solute present in 1 Lit. of solution. It…
Q: A chemist prepares a solution of silver(I) nitrate (AGNO,) by measuring out 245. µmol of silver(I)…
A: Concentration in terms of Micromole per litre...
Q: O CHEMICAL REACTIONS Using molarity to find solute mass and solution volume A chemist adds 1.60 L of…
A: A chemist adds 1.60 L of a 0.0014 mM copper(II) fluoride (CuF₂) solution to a reaction flask.…
Q: A chemist prepares a solution of copper(II) sulfate (CuSO,) by measuring out 16. umol of copper(II)…
A: Volume =250 ml = 0.250 L ( 1 L = 1000ml ) Mole = 16 micromole
Q: A chemist prepares a solution of silver nitrate (AGNO,) by measuring out 0.752 kg of silver nitrate…
A: Given : Mass of AgNO3 mixed = 0.752 Kg = 752 g (Since 1 Kg = 1000 g) And…
Q: A chemist prepares a solution of magnesium fluoride (MgF,) by measuring out 0.032 µmol of magnesium…
A: Concentration of a solution is the ratio of moles of solute to volume of solution.
Q: A chemist prepares a solution of calcium sulfate (CASO) by weighing out 0.06781 g of calcium sulfate…
A:
Q: A chemist adds 55.0 mL of a 1.4M barium acetate (Ba(C,H,0,)) solution to a reaction flask. Calculate…
A: Given, Volume of Barium Acetate = 55 mL = 0.055 L Molarity of Barium Acetate = 1.4M
Q: The mass in milligrams of oxygen (molar mass= 16 g/mol) in 1920 cm³ of 1.76X10-3 M solution of…
A:
Q: A chemist prepares a solution of iron(III) bromide (FeBr,) by measuring out 7.3 µmol of iron(III)…
A: Molarity : Moles of solute present per liter of solution is called molarity of that solution.…
Q: A chemist prepares a solution of silver(I) nitrate (AGNO,) by measuring out 107. µmol of silver(1)…
A: Given data contains, Moles of silver nitrate is 107 μmol=107 μmol×1 mmole1000 μmol=0.107 mmol Volume…
Q: (H₂O). Aqueous hydrochloric acid (HCI) will react with solid sodium hydroxide (NaOH) to produce…
A: Given-> Weight of HCl = 0.729 gm Weight of NaOH = 0.44 gm
Q: Aqueous hydrochloric acid (HCI) will react with solid sodium hydroxide (NaOH) to produce aqueous…
A:
Q: Aqueous hydrochloric acid (HCI) will react with solid sodium hydroxide (NaOH) to produce aqueous…
A:
Q: A chemist prepares a solution of silver(I) nitrate (AGNO,) by measuring out 413. umol of silver(I)…
A:
Q: A particular brand of beef jerky contains 6.05x10-2% sodium nitrite by mass and is sold in an…
A: Given - Mass % sodium nitrite (NaNO2) =6.05×10-2% Mass of bag = 8.00 oz
Q: A chemist prepares a solution of magnesium fluoride (MgF,) by measuring out 0.0152 µmol of magnesium…
A: Given that, Moles of MgF2 added to 200 mL(= 0.2 L) flask = 0.0152 µmol = 0.0152×10-6 mol
Q: A chemist adds 450.0 mL of a 1.0 mol/L barium acetate (Ba (C,H,0,),) solution to a reaction flask.…
A: Volume of solution = 450.0 ml ...Or because 1L = 1000 ml ....so, 450.0 ml × (1L / 1000 ml ) =…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- How many mL of 0.556 molar magnesium chloride must a scientist pour into a beaker to have 17.6 g of magnesium chloride in the beaker? Put answer in box in regular non scientific notation with one past decimal.A chemist must dilute 16.6mL of 2.03M aqueous zinc nitrate ZnNO32 solution until the concentration falls to 1.00M. She'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in milliliters. Round your answer to 3 significant digits.Aqueous hydrochloric acid (HCI) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H₂O). Suppose 32.4 g of hydrochloric acid is mixed with 63. g of sodium hydroxide. Calculate the maximum mass of sodium chloride that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. g 10 X IEE olo Ar (Please type answer no write by hend)
- Aqueous hydrobromic acid HBr will react with solid sodium hydroxide NaOH to produce aqueous sodium bromide NaBr and liquid water H2O. Suppose 1.62 g of hydrobromic acid is mixed with 0.45 g of sodium hydroxide. Calculate the maximum mass of sodium bromide that could be produced by the chemical reaction. Round your answer to 2 significant digits.A chemist must dilute 14.9mL of 1.88M aqueous sodium carbonate (Na2CO3) solution until the concentration falls to 1.00M. He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in milliliters. Round your answer to 3 significant digits.A chemist must prepare 300.mL of 2.00M aqueous silver perchlorate AgClO4 working solution. He'll do this by pouring out some 5.62molL aqueous silver perchlorate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the silver perchlorate stock solution that the chemist should pour out. Round your answer to 3 significant digits.
- An aqueous stock solution is 85.0% H3PO4 by mass and its density is 1.69 g/mL. What volume of this solution is required to make 1.25 L of 2.55 mol/L H3PO4(aq)? Give your answer in millilitres, accurate to three significant figures. Enter the correct symbol for millilitres in the units box.the us national institute of standards and technology used mercury gauge as a primary standard for pressure until is was decommissioned in 2019. the device was 3.00 meters tall and contained 225 kilograms of mercury. if the density of mercury is 13.6 g/ml, what was the volume of mercury contained in this device in liters?A chemist prepares a solution of silver(I) nitrate AgNO3 by measuring out 6.4102 x 10^2 μmol of silver(I) nitrate into a 450.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's silver(I) nitrate solution. Round your answer to 2 significant digits.
- Aqueous hydrobromic acid HBr will react with solid sodium hydroxide NaOH to produce aqueous sodium bromide NaBr and liquid water H2O . Suppose 61. g of hydrobromic acid is mixed with 57.3 g of sodium hydroxide. Calculate the maximum mass of sodium bromide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.Methanol (CH4O) has a density of 0.791 gmLgmL and a molar mass of 32.05 gmolgmol. How many moles of methanol are in a 0.834 mL (milliliter) sample of methanol? Report your answer rounded to the proper number of significant figures.Aqueous hydrobromic acid (HBr) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H_2O). Suppose 47. g of hydrobromic acid is mixed with 38.5 g of sodium hydroxide. Calculate the minimum mass of hydrobromic add that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.

