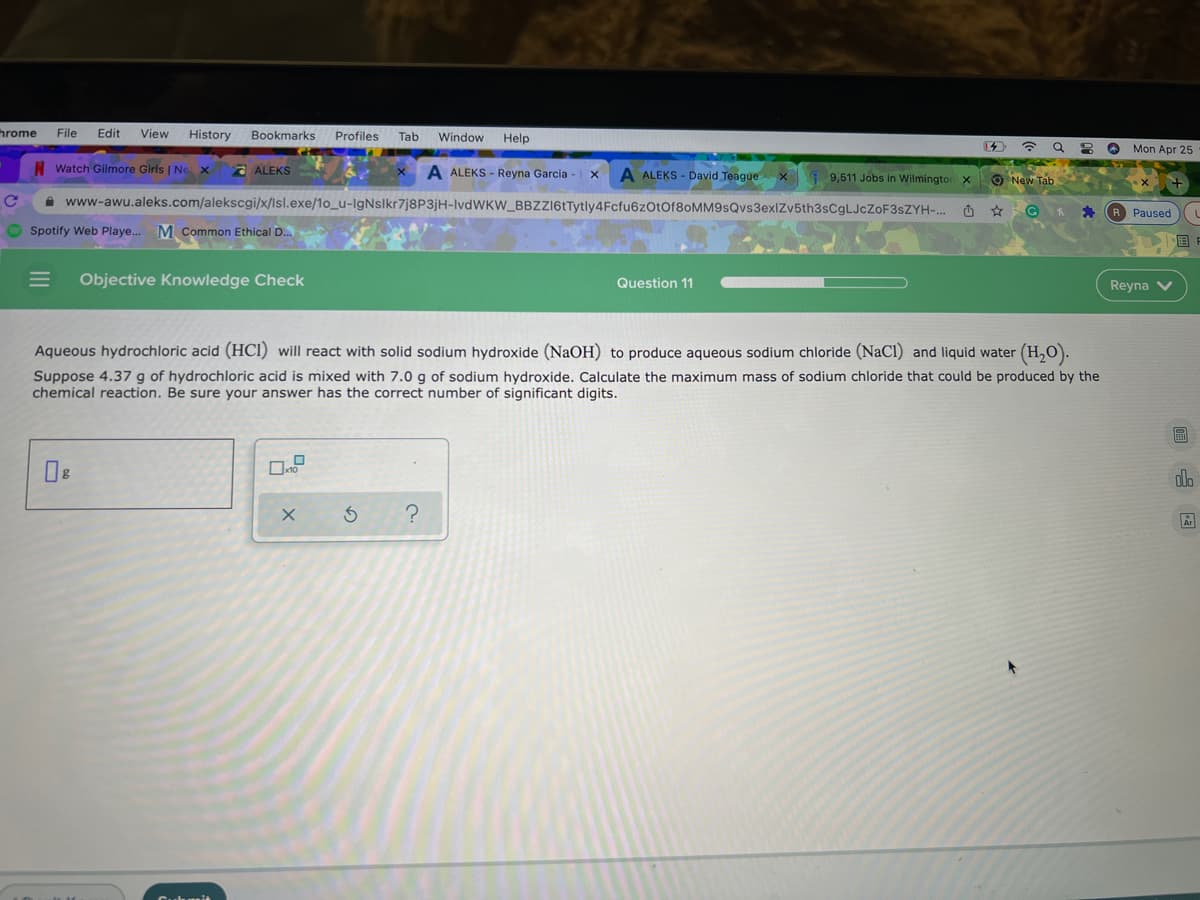
Aqueous hydrochloric acid (HCI) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCI) and liquid water (H,O). Suppose 4.37 g of hydrochloric acid is mixed with 7.0 g of sodium hydroxide. Calculate the maximum mass of sodium chloride that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.
Aqueous hydrochloric acid (HCI) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCI) and liquid water (H,O). Suppose 4.37 g of hydrochloric acid is mixed with 7.0 g of sodium hydroxide. Calculate the maximum mass of sodium chloride that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 49A
Related questions
Question
Transcribed Image Text:hrome
File
Edit
View
History
Bookmarks
Profiles
Tab
Window Help
令Q
2 O Mon Apr 25
Watch Gilmore Girls | Ne x
a ALEKS
A ALEKS - Reyna Garcia
A ALEKS - David Teague
9,511 Jobs in Wilmington x
O New Tab
A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH-lvdWKW BBZZ16tTytly4Fcfu6zOtOf8oMM9sQvs3exlZv5th3sCgLJcZoF3sZYH-. O
R Paused
O Spotify Web Playe.. M Common Ethical D..
国
Objective Knowledge Check
Question 11
Reyna V
Aqueous hydrochloric acid (HCl) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H,O).
Suppose 4.37 g of hydrochloric acid is mixed with 7.0 g of sodium hydroxide. Calculate the maximum mass of sodium chloride that could be produced by the
chemical reaction. Be sure your answer has the correct number of significant digits.
圖
dlo
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning
