O Consider this acid-base reaction; CH3 CH₂ NH3 + H₂P CH3 = a) Draw the products. b) Draw the electron arrows for the forward reaction. c) Is the reactant acid or the product acid stronger? Explain structurally, not with Ka values.. d) Is AG for the forward reaction positive or negative?
O Consider this acid-base reaction; CH3 CH₂ NH3 + H₂P CH3 = a) Draw the products. b) Draw the electron arrows for the forward reaction. c) Is the reactant acid or the product acid stronger? Explain structurally, not with Ka values.. d) Is AG for the forward reaction positive or negative?
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter4: Acids And Bases
Section: Chapter Questions
Problem 4.33P: Complete the equation for the reaction between each Lewis acid-base pair. In each equation, label...
Related questions
Question
stuck on this one
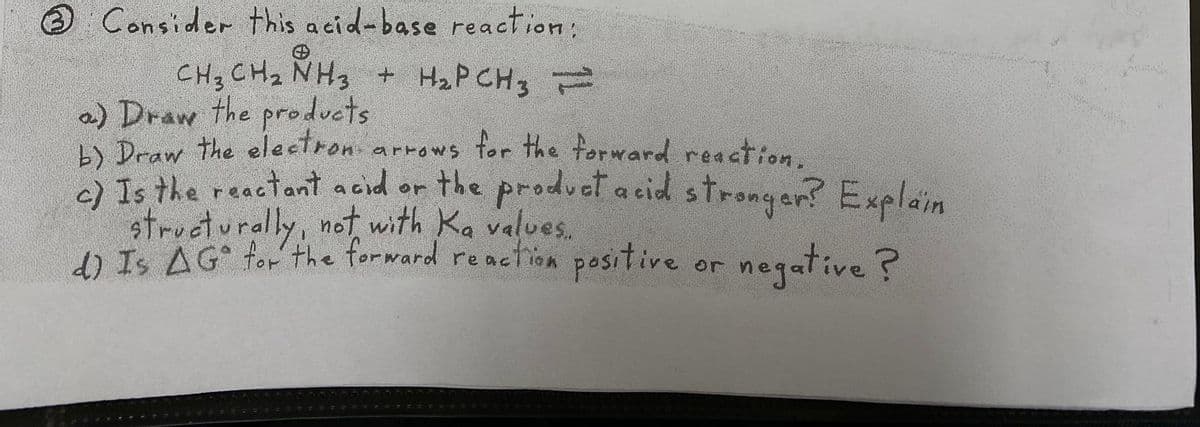
Transcribed Image Text:Consider this acid-base reaction:
CH3 CHz NH3 + H2P CH3
a) Draw the products
L) Draw the electron arrows for the forward reaction.
2ls the reactant acid or the produet a cid strongan? Explain
structurally, not with Ka values.
d) Is AG for the forward
reaction
positive
negative ?
or
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning