O ELECTROCHEMISTRY Writing the half-reactions of a single-... AA Study this chemical reaction: FeSO4(aq) +Zn(s) → Fe(S)+ZnSO4(aq) oxidation: reduction: Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction. ローロ www-awa.aleks.com e 0/5 On Andre
O ELECTROCHEMISTRY Writing the half-reactions of a single-... AA Study this chemical reaction: FeSO4(aq) +Zn(s) → Fe(S)+ZnSO4(aq) oxidation: reduction: Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction. ローロ www-awa.aleks.com e 0/5 On Andre
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
100%
The 2nd picture is an example of Alex’s answer format

Transcribed Image Text:2+
Ca(s) Ca²+ (aq) +2e
2+
• The reactant being reduced is z
electrons to form neutral zinc ato
describes this:
2+
Zn²+ (aq) +2e
Zn(S)
Here is the completed table:
EANSWER
2+
oxidation: Ca(s) → Ca“ (aq)+2e
2+
reduction: Zn(aq)+2e
Zn(s)
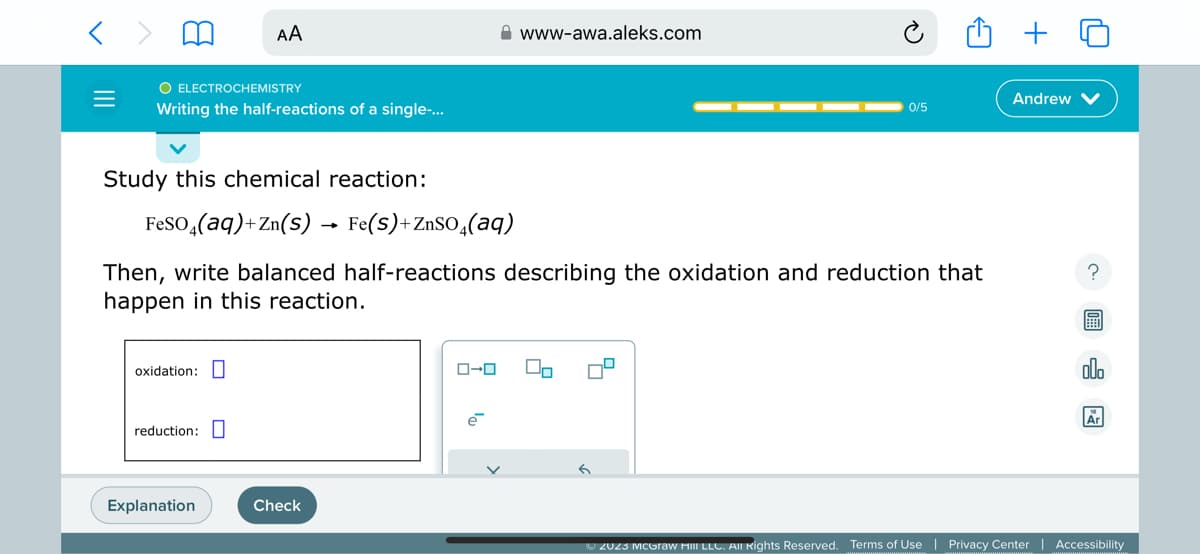
Transcribed Image Text:O ELECTROCHEMISTRY
Writing the half-reactions of a single-...
Study this chemical reaction:
AA
oxidation:
reduction:
FeSO4(aq)+Zn(s) → Fe(S)+ZnSO4(aq)
Then, write balanced half-reactions describing the oxidation and reduction that
happen in this reaction.
Explanation
Check
ローロ
www-awa.aleks.com
e
0/5
Andrew
?
A
olo
Ar
Ⓒ2023 McGraw HiIII LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning