o Which of the following solvents is the best to use for recrystallization of Solute A? а. Water b. Ethyl Acetate c. Diethyl Ether d. Hexane o Suppose that we use 50 mL of the best solvent (the one that gives the highest yield) to recrystallize 2.0 g of solute A, how many grams of solute A will be recovered? а. 1.6 g b. 1.8 g c. 0.80 g d. 0.90 g o Calculate for percent yield of solute A (initial mass = 2.00 g) if it is recrystallized using 50 mL of the best solvent (the one that gives the highest yield). a. 45% b. 40%
o Which of the following solvents is the best to use for recrystallization of Solute A? а. Water b. Ethyl Acetate c. Diethyl Ether d. Hexane o Suppose that we use 50 mL of the best solvent (the one that gives the highest yield) to recrystallize 2.0 g of solute A, how many grams of solute A will be recovered? а. 1.6 g b. 1.8 g c. 0.80 g d. 0.90 g o Calculate for percent yield of solute A (initial mass = 2.00 g) if it is recrystallized using 50 mL of the best solvent (the one that gives the highest yield). a. 45% b. 40%
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question
Hi! I need answers for numbers 2 and 3. My professor said that
The unit of solubility is in grams per 100mL, while the solvent used is only 50mL, so a yield of close to 2.0g is impossible. Recalculate using this information.
Thanks!
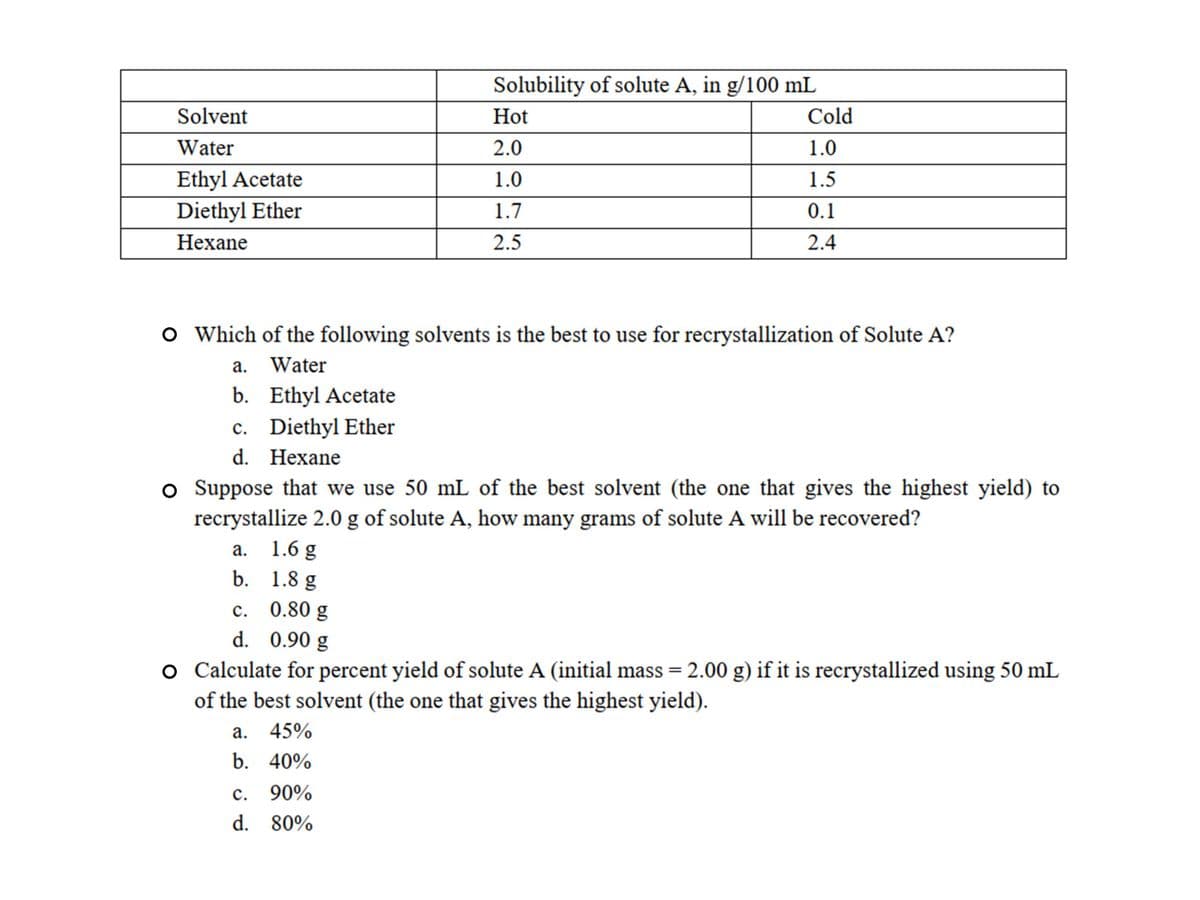
Transcribed Image Text:Solubility of solute A, in g/100 mL
Solvent
Hot
Cold
Water
2.0
1.0
Ethyl Acetate
1.0
1.5
Diethyl Ether
1.7
0.1
Нехane
2.5
2.4
O Which of the following solvents is the best to use for recrystallization of Solute A?
а.
Water
b. Ethyl Acetate
c. Diethyl Ether
d. Нехane
o Suppose that we use 50 mL of the best solvent (the one that gives the highest yield) to
recrystallize 2.0 g of solute A, how many grams of solute A will be recovered?
1.6 g
b. 1.8 g
а.
c. 0.80 g
d. 0.90 g
с.
o Calculate for percent yield of solute A (initial mass = 2.00 g) if it is recrystallized using 50 mL
of the best solvent (the one that gives the highest yield).
%3D
а.
45%
b. 40%
с.
90%
d. 80%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
